Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus
- PMID: 11074002
- PMCID: PMC86557
- DOI: 10.1128/MCB.20.23.9041-9054.2000
Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus
Abstract
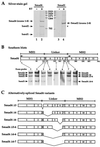
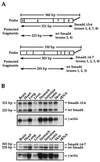
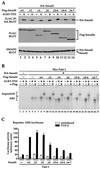
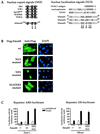
Smad4 plays a pivotal role in all transforming growth factor beta (TGF-beta) signaling pathways. Here we describe six widely expressed alternatively spliced variants of human Smad4 with deletions of different exons in the linker, the region of Smad4 that separates the two well-conserved MH1 and MH2 domains. All these Smad4 variants form complexes with activated Smad2 and Smad3 and are incorporated into DNA-binding complexes with the transcription factor Fast-1, regardless of the amount of linker they contain. However, sequences encoded by exons 5 to 7 in the linker are essential for transcriptional activation. Most importantly, our observation that different Smad4 isoforms have different subcellular localizations has led us to the identification of a functional CRM1-dependent nuclear export signal in the Smad4 linker and a constitutively active nuclear localization signal in the N-terminal MH1 domain. In the absence of TGF-beta signaling, we conclude that Smad4 is rapidly and continuously shuttling between the nucleus and the cytoplasm, the distribution of Smad4 between the nucleus and the cytoplasm being dictated by the relative strengths of the nuclear import and export signals. We demonstrate that inhibition of CRM1-mediated nuclear export by treatment of cells with leptomycin B results in endogenous Smad4 accumulating very rapidly in the nucleus. Endogenous Smad2 and Smad3 are completely unaffected by leptomycin B treatment, indicating that the nucleocytoplasmic shuttling is specific for Smad4. We propose that, upon TGF-beta signaling, complex formation between Smad4 and activated Smad2 or -3 leads to nuclear accumulation of Smad4 through inhibition of its nuclear export. We demonstrate that after prolonged TGF-beta signaling Smad2 becomes dephosphorylated and Smad2 and Smad4 accumulate back in the cytoplasm.
Figures





Similar articles
-
An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity.Oncogene. 2003 Feb 20;22(7):1057-69. doi: 10.1038/sj.onc.1206212. Oncogene. 2003. PMID: 12592392
-
Smad3 inhibits transforming growth factor-beta and activin signaling by competing with Smad4 for FAST-2 binding.J Biol Chem. 1999 Oct 29;274(44):31229-35. doi: 10.1074/jbc.274.44.31229. J Biol Chem. 1999. PMID: 10531318
-
Nuclear targeting of transforming growth factor-beta-activated Smad complexes.J Biol Chem. 2005 Jun 3;280(22):21329-36. doi: 10.1074/jbc.M500362200. Epub 2005 Mar 30. J Biol Chem. 2005. PMID: 15799969
-
The transcriptional role of Smads and FAST (FoxH1) in TGFbeta and activin signalling.Mol Cell Endocrinol. 2001 Jun 30;180(1-2):3-11. doi: 10.1016/s0303-7207(01)00524-x. Mol Cell Endocrinol. 2001. PMID: 11451566 Review.
-
Smads as transcriptional co-modulators.Curr Opin Cell Biol. 2000 Apr;12(2):235-43. doi: 10.1016/s0955-0674(99)00081-2. Curr Opin Cell Biol. 2000. PMID: 10712925 Review.
Cited by
-
Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics.Mol Cell Biol. 2009 May;29(9):2443-55. doi: 10.1128/MCB.01443-08. Epub 2009 Feb 17. Mol Cell Biol. 2009. PMID: 19223462 Free PMC article.
-
Post-translational regulation of TGF-β receptor and Smad signaling.FEBS Lett. 2012 Jul 4;586(14):1871-84. doi: 10.1016/j.febslet.2012.05.010. Epub 2012 May 19. FEBS Lett. 2012. PMID: 22617150 Free PMC article. Review.
-
Analysis of ligand-dependent nuclear accumulation of Smads in TGF-beta signaling.Methods Mol Biol. 2010;647:95-111. doi: 10.1007/978-1-60761-738-9_5. Methods Mol Biol. 2010. PMID: 20694662 Free PMC article.
-
SMAD4 and its role in pancreatic cancer.Tumour Biol. 2015 Jan;36(1):111-9. doi: 10.1007/s13277-014-2883-z. Epub 2014 Dec 3. Tumour Biol. 2015. PMID: 25464861 Review.
-
Decoding the quantitative nature of TGF-beta/Smad signaling.Trends Cell Biol. 2008 Sep;18(9):430-42. doi: 10.1016/j.tcb.2008.06.006. Epub 2008 Aug 15. Trends Cell Biol. 2008. PMID: 18706811 Free PMC article. Review.
References
-
- Chen C H, von Kessler D P, Park W, Wang B, Ma Y, Beachy P A. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–316. - PubMed
-
- Chen X, Rubock M J, Whitman M. A transcriptional partner for MAD proteins in TGF-β signalling. Nature. 1996;383:691–696. - PubMed
-
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. - PubMed
-
- de Caestecker M P, Hemmati P, Larisch-Bloch S, Ajmera R, Roberts A B, Lechleider R J. Characterization of functional domains within Smad4/DPC4. J Biol Chem. 1997;272:13690–13696. - PubMed
-
- de Caestecker M P, Yahata T, Wang D, Parks W T, Huang S, Hill C S, Shioda T, Roberts A B, Lechleider R J. The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem. 2000;275:2115–2122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
