Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons
- PMID: 11076963
- PMCID: PMC2169450
- DOI: 10.1083/jcb.151.4.779
Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons
Abstract
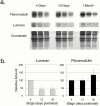
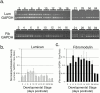
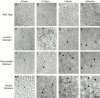
Collagen fibrillogenesis is finely regulated during development of tissue-specific extracellular matrices. The role(s) of a leucine-rich repeat protein subfamily in the regulation of fibrillogenesis during tendon development were defined. Lumican-, fibromodulin-, and double-deficient mice demonstrated disruptions in fibrillogenesis. With development, the amount of lumican decreases to barely detectable levels while fibromodulin increases significantly, and these changing patterns may regulate this process. Electron microscopic analysis demonstrated structural abnormalities in the fibrils and alterations in the progression through different assembly steps. In lumican-deficient tendons, alterations were observed early and the mature tendon was nearly normal. Fibromodulin-deficient tendons were comparable with the lumican-null in early developmental periods and acquired a severe phenotype by maturation. The double-deficient mice had a phenotype that was additive early and comparable with the fibromodulin-deficient mice at maturation. Therefore, lumican and fibromodulin both influence initial assembly of intermediates and the entry into fibril growth, while fibromodulin facilitates the progression through growth steps leading to mature fibrils. The observed increased ratio of fibromodulin to lumican and a competition for the same binding site could mediate these transitions. These studies indicate that lumican and fibromodulin have different developmental stage and leucine-rich repeat protein specific functions in the regulation of fibrillogenesis.
Figures




References
-
- Birk D.E., Hahn R.A., Linsenmayer C.Y., Zycband E.I. Characterization of fibril segments from chicken embryo cornea, dermis and tendon. Matrix Biol. 1996;15:111–118. - PubMed
-
- Birk D.E., Linsenmayer T.F. Collagen fibril assembly, depostion, and organization into tissue-specific matrices. In: Yurchenco P.D., Birk D.E., Mecham R.P., editors. Extracellular Matrix Assembly and Structure. Academic Press, Inc; New York, NY: 1994. pp. 91–128.
-
- Birk D.E., Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72:352–361. - PubMed
-
- Birk D.E., Nurminskaya M.V., Zycband E.I. Collagen fibrillogenesis in situfibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202:229–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

