c-Jun activation-dependent tumorigenic transformation induced paradoxically by overexpression or block of S-adenosylmethionine decarboxylase
- PMID: 11076965
- PMCID: PMC2169445
- DOI: 10.1083/jcb.151.4.801
c-Jun activation-dependent tumorigenic transformation induced paradoxically by overexpression or block of S-adenosylmethionine decarboxylase
Abstract
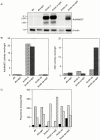
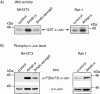
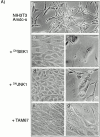
All mammalian cells absolutely require polyamines (putrescine, spermidine, and spermine) for growth. Here we show that the overexpression of cDNA for S-adenosylmethionine decarboxylase (AdoMetDC), the main regulatory enzyme in the biosynthesis of higher polyamines, induces transformation of rodent fibroblasts when expressed in the sense or the antisense orientation. Both transformants were able to induce invasive tumors in nude mice. Neither transformation was associated with activation of the mitogen-activated protein kinases Erk1 and Erk2. Instead, the AdoMet DC sense, but not antisense, transformants displayed constitutive activation of the c-Jun NH(2)-terminal kinase (JNK) pathway. However, both transformations converged on persistent phosphorylation of endogenous c-Jun at Ser73. The phenotype of the AdoMetDC sense transformants was reversed by expression of dominant-negative mutants of SEK1 (MKK4), JNK1, and c-Jun (TAM-67), which were also found to impair cytokinesis. Similarly, TAM-67 reverted the morphology of the AdoMetDC-antisense expressors. This report is the first demonstration of a protein whose overexpression or block of synthesis can induce cell transformation. In addition, we show that the polyamine biosynthetic enzymes require c-Jun activation for eliciting their biological effects.
Figures




References
-
- Alessandrini A., Greulich H., Huang W., Erikson R.L. Mek1 phosphorylation site mutants activate Raf-1 in NIH3T3 cells. J. Biol. Chem. 1996;271:31612–31618. - PubMed
-
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R.J., Rahmsdorf H.J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. - PubMed
-
- Attar B.M., Atten M.J., Holian O. MAPK activity is down-regulated in human colon adenocarcinomacorrelation with PKC activity. Anticancer Res. 1996;16:395–400. - PubMed
-
- Auvinen M., Paasinen A., Andersson L.C., Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992;360:355–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

