Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors
- PMID: 11076970
- PMCID: PMC2169436
- DOI: 10.1083/jcb.151.4.863
Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors
Abstract
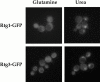
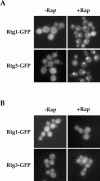
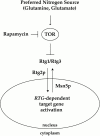
De novo biosynthesis of amino acids uses intermediates provided by the TCA cycle that must be replenished by anaplerotic reactions to maintain the respiratory competency of the cell. Genome-wide expression analyses in Saccharomyces cerevisiae reveal that many of the genes involved in these reactions are repressed in the presence of the preferred nitrogen sources glutamine or glutamate. Expression of these genes in media containing urea or ammonia as a sole nitrogen source requires the heterodimeric bZip transcription factors Rtg1 and Rtg3 and correlates with a redistribution of the Rtg1p/Rtg3 complex from a predominantly cytoplasmic to a predominantly nuclear location. Nuclear import of the complex requires the cytoplasmic protein Rtg2, a previously identified upstream regulator of Rtg1 and Rtg3, whereas export requires the importin-beta-family member Msn5. Remarkably, nuclear accumulation of Rtg1/Rtg3, as well as expression of their target genes, is induced by addition of rapamycin, a specific inhibitor of the target of rapamycin (TOR) kinases. We demonstrate further that Rtg3 is a phosphoprotein and that its phosphorylation state changes after rapamycin treatment. Taken together, these results demonstrate that target of rapamycin signaling regulates specific anaplerotic reactions by coupling nitrogen quality to the activity and subcellular localization of distinct transcription factors.
Figures






References
-
- Beck T., Hall M.N. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. - PubMed
-
- Brachmann C.B., Davies A., Cost G.J., Caputo E., Li J., Hieter P., Boeke J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288Ca useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

