Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis
- PMID: 11076972
- PMCID: PMC2169434
- DOI: 10.1083/jcb.151.4.891
Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis
Abstract
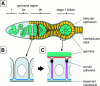
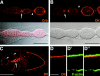
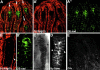
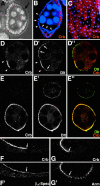
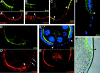
Analysis of the mechanisms that control epithelial polarization has revealed that cues for polarization are mediated by transmembrane proteins that operate at the apical, lateral, or basal surface of epithelial cells. Whereas for any given epithelial cell type only one or two polarization systems have been identified to date, we report here that the follicular epithelium in Drosophila ovaries uses three different polarization mechanisms, each operating at one of the three main epithelial surface domains. The follicular epithelium arises through a mesenchymal-epithelial transition. Contact with the basement membrane provides an initial polarization cue that leads to the formation of a basal membrane domain. Moreover, we use mosaic analysis to show that Crumbs (Crb) is required for the formation and maintenance of the follicular epithelium. Crb localizes to the apical membrane of follicle cells that is in contact with germline cells. Contact to the germline is required for the accumulation of Crb in follicle cells. Discs Lost (Dlt), a cytoplasmic PDZ domain protein that was shown to interact with the cytoplasmic tail of Crb, overlaps precisely in its distribution with Crb, as shown by immunoelectron microscopy. Crb localization depends on Dlt, whereas Dlt uses Crb-dependent and -independent mechanisms for apical targeting. Finally, we show that the cadherin-catenin complex is not required for the formation of the follicular epithelium, but only for its maintenance. Loss of cadherin-based adherens junctions caused by armadillo (beta-catenin) mutations results in a disruption of the lateral spectrin and actin cytoskeleton. Also Crb and the apical spectrin cytoskeleton are lost from armadillo mutant follicle cells. Together with previous data showing that Crb is required for the formation of a zonula adherens, these findings indicate a mutual dependency of apical and lateral polarization mechanisms.
Figures





References
-
- Baumgartner S., Littleton J.T., Broadie K., Bhat M.A., Harbecke R., Lengyel J.A., Chiquet-Ehrismann R., Prokop A., Bellen H.J. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. - PubMed
-
- Bhat M.A., Izaddoost S., Lu Y., Cho K.O., Choi K.W., Bellen H. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:633–645. - PubMed
-
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. - PubMed
-
- Brook W.J., Ostafichuk L.M., Piorecky J., Wilkinson M.D., Hodgetts D.J., Russell M.A. Gene expression during imaginal disc regeneration detected using enhancer-sensitive P-elements. Development (Camb.). 1993;117:1287–1297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

