The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers
- PMID: 11076974
- PMCID: PMC2169442
- DOI: 10.1083/jcb.151.4.919
The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers
Abstract
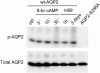
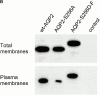
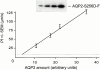
In renal principal cells, vasopressin regulates the shuttling of the aquaporin (AQP)2 water channel between intracellular vesicles and the apical plasma membrane. Vasopressin-induced phosphorylation of AQP2 at serine 256 (S256) by protein kinase A (PKA) is essential for its localization in the membrane. However, phosphorylated AQP2 (p-AQP2) has also been detected in intracellular vesicles of noninduced principal cells. As AQP2 is expressed as homotetramers, we hypothesized that the number of p-AQP2 monomers in a tetramer might be critical for the its steady state distribution. Expressed in oocytes, AQP2-S256D and AQP2-S256A mimicked p-AQP2 and non-p-AQP2, respectively, as routing and function of AQP2-S256D and wild-type AQP2 (wt-AQP2) were identical, whereas AQP2-S256A was retained intracellularly. In coinjection experiments, AQP2-S256A and AQP2-S256D formed heterotetramers. Coinjection of different ratios of AQP2-S256A and AQP2-S256D cRNAs revealed that minimally three AQP2-S256D monomers in an AQP2 tetramer were essential for its plasma membrane localization. Therefore, our results suggest that in principal cells, minimally three monomers per AQP2 tetramer have to be phosphorylated for its steady state localization in the apical membrane. As other multisubunit channels are also regulated by phosphorylation, it is anticipated that the stoichiometry of their phosphorylated and nonphosphorylated subunits may fine-tune the activity or subcellular localization of these complexes.
Figures





References
-
- Bao J., Alroy I., Waterman H., Schejter E.D., Brodie C., Gruenberg J., Yarden Y. Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J. Biol. Chem. 2000;275:26178–26186. - PubMed
-
- Brown D. Membrane recycling and epithelial cell function. Am. J. Physiol. 1989;256:F1–F12. - PubMed
-
- Brown D., Stow J.L. Protein trafficking and polarity in kidney epitheliumfrom cell biology to physiology. Physiol. Rev. 1996;76:245–297. - PubMed
-
- Brown D., Weyer P., Orci L. Vasopressin stimulates endocytosis in kidney collecting duct principal cells. Eur. J. Cell Biol. 1988;46:336–341. - PubMed
-
- Cheng C., Prince L.S., Snyder P.M., Welsh M.J. Assembly of the epithelial Na+ channel evaluated using sucrose gradient sedimentation analysis. J. Biol. Chem. 1998;273:22693–22700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

