Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1
- PMID: 11078524
- PMCID: PMC27228
- DOI: 10.1073/pnas.240347797
Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1
Abstract
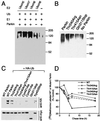
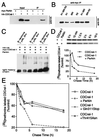
Parkinson's disease is a common neurodegenerative disorder in which familial-linked genes have provided novel insights into the pathogenesis of this disorder. Mutations in Parkin, a ring-finger-containing protein of unknown function, are implicated in the pathogenesis of autosomal recessive familial Parkinson's disease. Here, we show that Parkin binds to the E2 ubiquitin-conjugating human enzyme 8 (UbcH8) through its C-terminal ring-finger. Parkin has ubiquitin-protein ligase activity in the presence of UbcH8. Parkin also ubiquitinates itself and promotes its own degradation. We also identify and show that the synaptic vesicle-associated protein, CDCrel-1, interacts with Parkin through its ring-finger domains. Furthermore, Parkin ubiquitinates and promotes the degradation of CDCrel-1. Familial-linked mutations disrupt the ubiquitin-protein ligase function of Parkin and impair Parkin and CDCrel-1 degradation. These results suggest that Parkin functions as an E3 ubiquitin-protein ligase through its ring domains and that it may control protein levels via ubiquitination. The loss of Parkin's ubiquitin-protein ligase function in familial-linked mutations suggests that this may be the cause of familial autosomal recessive Parkinson's disease.
Figures


References
-
- Lang A E, Lozano A M. N Engl J Med. 1998;339:1044–1053. - PubMed
-
- Lang A E, Lozano A M. N Engl J Med. 1998;339:1130–1143. - PubMed
-
- Jenner P, Olanow C W. Ann Neurol. 1998;44:S72–S84. - PubMed
-
- Dunnett S B, Bjorklund A. Nature (London) 1999;399:A32–A39. - PubMed
-
- Pollanen M S, Dickson D W, Bergeron C. J Neuropathol Exp Neurol. 1993;52:183–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

