Meiotic lamin C2: the unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association
- PMID: 11078531
- PMCID: PMC27188
- DOI: 10.1073/pnas.240466597
Meiotic lamin C2: the unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association
Abstract
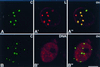
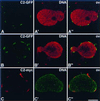
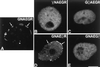
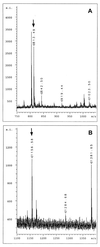
Meiotic lamin C2 is the only A-type lamin expressed during mammalian spermatogenesis. Typical for this short lamin is the unique hexapeptide GNAEGR, which substitutes the nonhelical amino terminus and part of the alpha-helical rod domain present in somatic lamins. Meiotic lamin C2 also lacks a carboxyl-terminal CaaX box, which is modified by isoprenylation and involved in nuclear envelope (NE) association of somatic isoforms. The mechanism by which lamin C2 becomes localized in the NE is totally unknown. Here we demonstrate that the hexapeptide GNAEGR is essential for this process: (i) Its deletion resulted in a diffuse distribution of lamin C2 within nuclei of transfected COS-7 cells; (ii) Mutated somatic lamin C, containing the sequence GNAEGR at its amino terminus, was located at the NE. The mass spectrometric analysis of the amino terminus of lamin C2 revealed that it is modified by myristoylation. Correspondingly, the substitution of the first glycine residue abolishes the NE association of lamin C2. We conclude that NE association of lamin C2 is achieved by a mechanism different from that of somatic lamins.
Figures


References
-
- Burke B, Gerace L. Cell. 1986;44:639–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

