Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex
- PMID: 11080149
- PMCID: PMC305826
- DOI: 10.1093/emboj/19.22.6020
Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex
Abstract
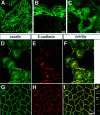
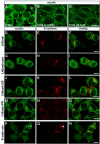
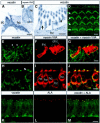
Defects in myosin VIIA are responsible for deafness in the human and mouse. The role of this unconventional myosin in the sensory hair cells of the inner ear is not yet understood. Here we show that the C-terminal FERM domain of myosin VIIA binds to a novel transmembrane protein, vezatin, which we identified by a yeast two-hybrid screen. Vezatin is a ubiquitous protein of adherens cell-cell junctions, where it interacts with both myosin VIIA and the cadherin-catenins complex. Its recruitment to adherens junctions implicates the C-terminal region of alpha-catenin. Taken together, these data suggest that myosin VIIA, anchored by vezatin to the cadherin-catenins complex, creates a tension force between adherens junctions and the actin cytoskeleton that is expected to strengthen cell-cell adhesion. In the inner ear sensory hair cells vezatin is, in addition, concentrated at another membrane-membrane interaction site, namely at the fibrillar links interconnecting the bases of adjacent stereocilia. In myosin VIIA-defective mutants, inactivity of the vezatin-myosin VIIA complex at both sites could account for splaying out of the hair cell stereocilia.
Figures




References
-
- Aberle H., Butz,S., Stappert,J., Weissig,H., Kemler,R. and Hoschuetzky,H. (1994) Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci., 107, 3655–3663. - PubMed
-
- Adams C.L. and Nelson,W.J. (1998) Cytomechanics of cadherin-mediated cell–cell adhesion. Curr. Opin. Cell Biol., 10, 572–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

