Euplotes telomerase contains an La motif protein produced by apparent translational frameshifting
- PMID: 11080168
- PMCID: PMC305813
- DOI: 10.1093/emboj/19.22.6230
Euplotes telomerase contains an La motif protein produced by apparent translational frameshifting
Abstract
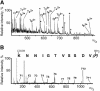
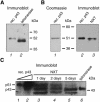
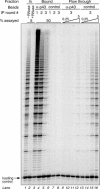
Telomerase is the ribonucleoprotein enzyme responsible for the replication of chromosome ends in most eukaryotes. In the ciliate Euplotes aediculatus, the protein p43 biochemically co-purifies with active telomerase and appears to be stoichiometric with both the RNA and the catalytic protein subunit of this telomerase complex. Here we describe cloning of the gene for p43 and present evidence that it is an authentic component of the telomerase holoenzyme. Comparison of the nucleotide sequence of the cloned gene with peptide sequences of the protein suggests that production of full-length p43 relies on a programmed ribosomal frameshift, an extremely rare translational mechanism. Anti-p43 antibodies immunodeplete telomerase RNA and telomerase activity from E.aediculatus nuclear extracts, indicating that the vast majority of mature telomerase complexes in the cell are associated with p43. The sequence of p43 reveals similarity to the La autoantigen, an RNA-binding protein involved in maturation of RNA polymerase III transcripts, and recombinant p43 binds telomerase RNA in vitro. By analogy to other La proteins, p43 may function in chaperoning the assembly and/or facilitating nuclear retention of telomerase.
Figures

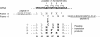



References
-
- Bandziulis R.J., Swanson,M.S. and Dreyfuss,G. (1989) RNA-binding proteins as developmental regulators. Genes Dev., 3, 431–437. - PubMed
-
- Beattie T.L., Zhou,W., Robinson,M.O. and Harrington,L. (1998) Reconstitution of human telomerase activity in vitro. Curr. Biol., 8, 177–180. - PubMed
-
- Blasco M.A, Lee,H.-W., Hande,M.P., Samper,E., Lansdorp,P.M., DePinho,R.E. and Greider,C.W. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell, 91, 25–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

