Oxygen-sensing persistent sodium channels in rat hippocampus
- PMID: 11080255
- PMCID: PMC2270177
- DOI: 10.1111/j.1469-7793.2000.00107.x
Oxygen-sensing persistent sodium channels in rat hippocampus
Abstract
1. Persistent sodium channel activity was recorded before and during hypoxia from cell-attached and inside-out patches obtained from cultured hippocampal neurons at a pipette potential (Vp) of +30 mV. Average mean current (IU) of these channels was very low under normoxic conditions and was similar in cell-attached and excised inside-out patches (-0.018 +/- 0.010 and -0.025 +/- 0.008 pA, respectively, n = 24). 2. Hypoxia increased the activity of persistent sodium channels in 10 cell-attached patches (IU increased from -0. 026 +/- 0.016 pA in control to -0.156 +/- 0.034 pA during hypoxia, n = 4, P = 0.013). The increased persistent sodium channel activity was most prominent at a VP between +70 and +30 mV (membrane potential, Vm = -70 to -30 mV) and could be blocked by lidocaine, TTX or R56865 (n = 5). Sodium cyanide (NaCN, 5 mM; 0.5-5 min) increased persistent sodium channel activity in cell-attached patches (n = 3) in a similar manner. 3. Hypoxia also increased sodium channel activity in inside-out patches from hippocampal neurons. Within 2-4 min of exposure to hypoxia, I had increased 9-fold to -0. 18 +/- 0.04 pA (n = 21, P = 0.001). Sodium channel activity increased further with longer exposures to hypoxia. 4. The hypoxia-induced sodium channel activity in inside-out patches could be inhibited by exposure to 10-100 microM lidocaine applied via the bath solution (I = -0.03 +/- 0.01 pA, n = 8) or by perfusion of the pipette tip with 1 microM TTX (I = -0.01 +/- 0.01 pA, n = 3). 5. The reducing agent dithiothreitol (DTT, 2-5 mM) rapidly abolished the increase in sodium channel activity caused by hypoxia in excised patches (I = -0.01 +/- 0.01 pA, n = 4). Similarly, reduced glutathione (GSH, 5-20 mM) also reversed the hypoxia-induced increase in sodium channel activity (IU = -0.02 +/- 0.02 pA, n = 5). 6. These results suggest that persistent sodium channels in neurons can sense O2 levels in excised patches of plasma membrane. Hypoxia triggers an increase in sodium channel activity. The redox reaction involved in increasing the sodium channel activity probably occurs in an auxiliary regulatory protein, co-localized in the plasma membrane.
Figures



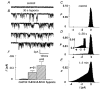
 ). Histograms show mean data and the vertical bars ±1
). Histograms show mean data and the vertical bars ±1 
 ), hypoxia (□) and exposure to TTX (▪).
), hypoxia (□) and exposure to TTX (▪).
 ), hypoxia (□) and exposure to lidocaine (▪, n = 8).
), hypoxia (□) and exposure to lidocaine (▪, n = 8).
 ), hypoxia (hypo, □) and exposure to drugs (D, DTT, n = 4; E, GSH, n = 5; ▪).
), hypoxia (hypo, □) and exposure to drugs (D, DTT, n = 4; E, GSH, n = 5; ▪).References
-
- Arden SR, Sinor JD, Potthoff WK, Aizenman E. Subunit-specific interactions of cyanide with the N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1998;273:21505–21511. - PubMed
-
- Astrup A, Sovsted P, Gjerris F, Sorensen HR. Increase in extracellular potassium in the brain during circulatory arrest: effects of hypothermia, lidocaine, and thiopental. Anesthesiology. 1981;55:256–262. - PubMed
-
- Boening JA, Kass IS, Cottrell JE, Chambers G. The effect of blocking sodium influx on anoxic damage in the rat hippocampal slice. Neuroscience. 1989;33:263–268. - PubMed
-
- Cherubini E, Ben-Ari Y, Krnjevic K. Anoxia produces smaller changes in synaptic transmission, membrane potential and input resistance in immature rat hippocampus. Journal of Neurophysiology. 1989;62:882–895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

