Molecular determinant for run-down of L-type Ca2+ channels localized in the carboxyl terminus of the 1C subunit
- PMID: 11080256
- PMCID: PMC2270188
- DOI: 10.1111/j.1469-7793.2000.00119.x
Molecular determinant for run-down of L-type Ca2+ channels localized in the carboxyl terminus of the 1C subunit
Abstract
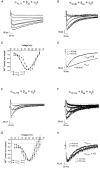
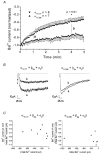
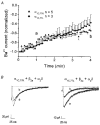
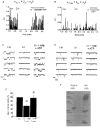
1. The role of the sequence 1572-1651 in the C-terminal tail of the alpha1C subunit in run-down of Ca2+ channels was studied by comparing functional properties of the conventional alpha1C,77 channel with those of three isoforms carrying alterations in this motif. 2. The pore-forming alpha1C subunits were co-expressed with alpha2delta and beta2a subunits in HEK-tsA201 cells, a subclone of the human embryonic kidney cell line, and studied by whole-cell and single-channel patch-clamp techniques. 3. Replacement of amino acids 1572-1651 in alpha1C,77 with 81 different amino acids leading to alpha1C,86 significantly altered run-down behaviour. Run-down of Ba2+ currents was rapid with alpha1C,77 channels, but was slow with alpha1C,86. 4. Transfer of the alpha1C,86 segments L (amino acids 1572-1598) or K (amino acids 1595-1652) into the alpha1C,77 channel yielded alpha1C,77L and alpha1C,77K channels, respectively, the run-down of which resembled more that of alpha1C,77. These results demonstrate that a large stretch of sequence between residues 1572 and 1652 of alpha1C,86 renders Ca2+ channels markedly resistant to run-down. 5. The protease inhibitor calpastatin added together with ATP was able to reverse the run-down of alpha1C,77 channels. Calpastatin expression was demonstrated in the HEK-tsA cells by Western blot analysis. 6. These results indicate a significant role of the C-terminal sequence 1572-1651 of the alpha1C subunit in run-down of L-type Ca2+ channels and suggest this sequence as a target site for a modulatory effect by endogenous calpastatin.
Figures


References
-
- Belles B, Hescheler J, Trautwein W, Blomgren K, Karlsson JO. A possible physiological role of the Ca-dependent protease calpain and its inhibitor calpastatin on the Ca2+ current in guinea pig myocytes. Pflügers Archiv. 1988a;412:554–556. - PubMed
-
- Belles B, Malecot CO, Hescheler J, Trautwein W. Run-down’ of the Ca current during whole-cell recordings in guinea pig heart cells: role of phosporylation and intracellular Ca2+ Pflügers Archiv. 1988b;411:353–360. - PubMed
-
- Catterall WA. Structure and function of voltage-gated ion channels. Annual Review of Biochemistry. 1995;64:493–531. - PubMed
-
- Cavalie A, Ochi R, Pelzer D, Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflügers Archiv. 1983;398:284–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

