Protein kinase C activates non-capacitative calcium entry in human platelets
- PMID: 11080259
- PMCID: PMC2270184
- DOI: 10.1111/j.1469-7793.2000.00159.x
Protein kinase C activates non-capacitative calcium entry in human platelets
Abstract
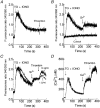
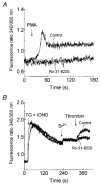
1. In many non-excitable cells Ca2+ influx is mainly controlled by the filling state of the intracellular Ca2+ stores. It has been suggested that this store-mediated or capacitative Ca2+ entry is brought about by a physical and reversible coupling of the endoplasmic reticulum with the plasma membrane. Here we provide evidence for an additional, non-capacitative Ca2+ entry mechanism in human platelets. 2. Changes in cytosolic Ca2+ and Sr2+ were measured in human platelets loaded with the fluorescent indicator fura-2. 3. Depletion of the internal Ca2+ stores with thapsigargin plus a low concentration of ionomycin stimulated store-mediated cation entry, as demonstrated upon Ca2+ or Sr2+ addition. Subsequent treatment with thrombin stimulated further divalent cation entry in a concentration-dependent manner. 4. Direct activation of protein kinase C (PKC) by phorbol-12-myristate-13-acetate or 1-oleoyl-2-acetyl-sn-glycerol also stimulated divalent cation entry, without evoking the release of Ca2+ from intracellular stores. Cation entry evoked by thrombin or activators of PKC was abolished by the PKC inhibitor Ro-31-8220. 5. Unlike store-mediated Ca2+ entry, jasplakinolide, which reorganises actin filaments into a tight cortical layer adjacent to the plasma membrane, did not inhibit divalent cation influx evoked by thrombin when applied after Ca2+ store depletion, or by activators of PKC. Thrombin also activated Ca2+ entry in platelets in which the release from intracellular stores and store-mediated Ca2+ entry were blocked by xestospongin C. 6. These results indicate that the non-capacitative divalent cation entry pathway is regulated independently of store-mediated entry and does not require coupling of the endoplasmic reticulum and the plasma membrane. These results support the existence of a mechanism for receptor-evoked Ca2+ entry in human platelets that is independent of Ca2+ store depletion. This Ca2+ entry mechanism may be activated by occupation of G-protein-coupled receptors, which activate PKC, or by direct activation of PKC, thus generating non-capacitative Ca2+ entry alongside that evoked following the release of Ca2+ from the intracellular stores.
Figures






References
-
- Clementi E, Scheer H, Zacchetti D, Fasolato C, Pozzan T, Meldolesi J. Two separate plasma membrane Ca2+ carriers participate in receptor-mediated Ca2+ influx in rat hepatocytes. Journal of Biological Chemistry. 1992;267:2164–2172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

