Cell surface expansion in polarly growing root hairs of Medicago truncatula
- PMID: 11080274
- PMCID: PMC59196
- DOI: 10.1104/pp.124.3.959
Cell surface expansion in polarly growing root hairs of Medicago truncatula
Abstract
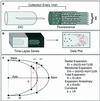
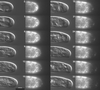
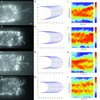
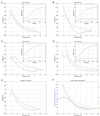
Fluorescent microspheres were used as material markers to investigate the relative rates of cell surface expansion at the growing tips of Medicago truncatula root hairs. From the analysis of tip shape and microsphere movements, we propose three characteristic zones of expansion in growing root hairs. The center of the apical dome is an area of 1- to 2- microm diameter with relatively constant curvature and high growth rate. Distal to the apex is a more rapidly expanding region 1 to 2 microm in width exhibiting constant surges of off-axis growth. This middle region forms an annulus of maximum growth rate and is visible as an area of accentuated curvature in the tip profile. The remainder of the apical dome is characterized by strong radial expansion anisotropy where the meridional rate of expansion falls below the radial expansion rate. Data also suggest possible meridional contraction at the juncture between the apical dome and the cell body. The cell cylinder distal to the tip expands slightly over time, but only around the circumference. These data for surface expansion in the legume root hair provide new insight into the mechanism of tip growth and the morphogenesis of the root hair.
Figures


References
-
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Hofte H, Plazanski J, Birch R, Cork A, Glover J, Redmond J, Williamson RE. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. - PubMed
-
- Bartnicki-Garcia S, Bartnicki DD, Gierz G. Determinants of fungal cell wall morphology: the vesicle supply center. Can J Bot. 1995;73:S372–S378.
-
- Bibikova T, Zhigilei A, Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203:495–505. - PubMed
-
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the cell during growth. Plant J. 1993;3:1–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

