Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin
- PMID: 11080299
- PMCID: PMC59221
- DOI: 10.1104/pp.124.3.1229
Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin
Abstract
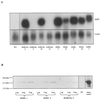
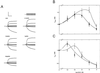
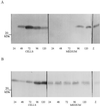
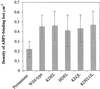
To explore the role of auxin-binding protein (ABP1) in planta, a number of transgenic tobacco (Nicotiana tabacum) lines were generated. The wild-type KDEL endoplasmic reticulum targeting signal was mutated to HDEL, another common retention sequence in plants, and to KEQL or KDELGL to compromise its activity. The auxin-binding kinetics of these forms of ABP1 were found to be similar to those of ABP1 purified from maize (Zea mays). To test for a physiological response mediated by auxin, intact guard cells of the transgenic plants were impaled with double-barreled microelectrodes, and auxin-dependent changes in K(+) currents were recorded under voltage clamp. Exogenous auxin affected inwardly and outwardly rectifying K(+) currents in a dose-dependent manner. Auxin sensitivity was markedly enhanced in all plants overexpressing ABP1, irrespective of the form present. Immunogold electron microscopy was used to investigate the localization of ABP1 in the transgenic plants. All forms were detected in the endoplasmic reticulum and the KEQL and KDELGL forms passed further across the Golgi stacks than KDEL and HDEL forms. However, neither electron microscopy nor silver-enhanced immunogold epipolarization microscopy revealed differences in cell surface ABP1 abundance for any of the plants, including control plants, which indicated that overexpression of ABP1 alone was sufficient to confer increased sensitivity to added auxin. Jones et al. ([1998] Science 282: 1114-1117) found increased cell expansion in transgenic plants overexpressing wild-type ABP1. Single cell recordings extend this observation, with the demonstration that the auxin sensitivity of guard cell K(+) currents is mediated, at least in part, by ABP1.
Figures


References
-
- Andres DA, Rhodes JD, Meisel RL, Dixon JE. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991;266:14277–14282. - PubMed
-
- Barbier-Brygoo H, Ephritikine G, Klämbt D, Maurel C, Palme K, Schell J, Guern J. Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J. 1991;1:83–93. - PubMed
-
- Barbier-Brygoo H, Zimmermann S, Thomine S, White IR, Millner P, Guern J. Elementary response chains at the plasma membrane involve external ABP1 and multiple electrogenic ion transport proteins. Pant Growth Regul. 1996;18:23–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

