The role of vacuolar malate-transport capacity in crassulacean acid metabolism and nitrate nutrition. Higher malate-transport capacity in ice plant after crassulacean acid metabolism-induction and in tobacco under nitrate nutrition
- PMID: 11080309
- PMCID: PMC59231
- DOI: 10.1104/pp.124.3.1335
The role of vacuolar malate-transport capacity in crassulacean acid metabolism and nitrate nutrition. Higher malate-transport capacity in ice plant after crassulacean acid metabolism-induction and in tobacco under nitrate nutrition
Abstract
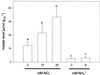
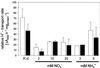
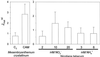
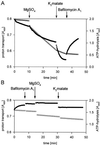
Anion uptake by isolated tonoplast vesicles was recorded indirectly via increased H(+)-transport by H(+)-pumping of the V-ATPase due to dissipation of the electrical component of the electrochemical proton gradient, Deltamu(H+), across the membrane. ATP hydrolysis by the V-ATPase was measured simultaneously after the Palmgren test. Normalizing for ATP-hydrolysis and effects of chloride, which was added to the assays as a stimulating effector of the V-ATPase, a parameter, J(mal)(rel), of apparent ATP-dependent malate-stimulated H(+)-transport was worked out as an indirect measure of malate transport capacity. This allowed comparison of various species and physiological conditions. J(mal)(rel) was high in the obligate crassulacean acid metabolism (CAM) species Kalanchoë daigremontiana Hamet et Perrier, it increased substantially after CAM induction in ice plant (Mesembryanthemum crystallinum), and it was positively correlated with NO(3)(-) nutrition in tobacco (Nicotiana tabacum). For tobacco this was confirmed by measurements of malate transport energized via the V-PPase. In ice plant a new polypeptide of 32-kD apparent molecular mass appeared, and a 33-kD polypeptide showed higher levels after CAM induction under conditions of higher J(mal)(rel). It is concluded that tonoplast malate transport capacity plays an important role in physiological regulation in CAM and NO(3)(-) nutrition and that a putative malate transporter must be within the 32- to 33-kD polypeptide fraction of tonoplast proteins.
Figures






References
-
- Allen S, Raven JA. Intracellular pH regulation in Ricinus communis grown with ammonium or nitrate as N source: the role of long distance transport. J Exp Bot. 1987;38:580–596.
-
- Ames B. Assay of inorganic phosphate, total phosphate and phosphatase. Methods Enzymol. 1966;8:115–118.
-
- Bennett A, Spanswick R. Optical measurement of ΔpH and ΔΨ in corn root membrane vesicles: kinetic analysis of Cl− effects on proton translocating ATPase. J Membr Biol. 1983;71:95–107.
-
- Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal Biochem. 1984;136:175–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

