Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots
- PMID: 11080310
- PMCID: PMC59232
- DOI: 10.1104/pp.124.3.1349
Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots
Abstract
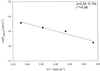
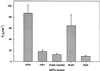
Boron is an essential micronutrient for plant growth and the boron content of plants differs greatly, but the mechanism(s) of its uptake into cells is not known. Boron is present in the soil solution as boric acid and it is in this form that it enters the roots. We determined the boron permeability coefficient of purified plasma membrane vesicles obtained from squash (Cucurbita pepo) roots and found it to be 3 x 10(-7) +/-1.4 x 10(-8) cm s(-1), six times higher than the permeability of microsomal vesicles. Boric acid permeation of the plasma membrane vesicles was partially inhibited (30%-39%) by mercuric chloride and phloretin, a non-specific channel blocker. The inhibition by mercuric chloride was readily reversible by 2-mercaptoethanol. The energy of activation for boron transport into the plasma membrane vesicles was 10.2 kcal mol(-1). Together these data indicate that boron enters plant cells in part by passive diffusion through the lipid bilayer of the plasma membrane and in part through proteinaceous channels. Expression of the major intrinsic protein (MIP) PIP1 in Xenopus laevis oocytes resulted in a 30% increase in the boron permeability of the oocytes. Other MIPs tested (PIP3, MLM1, and GlpF) did not have this effect. We postulate that certain MIPs, like those that have recently been shown to transport small neutral solutes, may also be the channels through which boron enters plant cells.
Figures

Similar articles
-
Permeability and the mechanism of transport of boric acid across the plasma membrane of Xenopus laevis oocytes.Biol Trace Elem Res. 2001 Aug;81(2):127-39. doi: 10.1385/BTER:81:2:127. Biol Trace Elem Res. 2001. PMID: 11554394
-
Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes.J Biol Chem. 1994 Apr 22;269(16):11869-72. J Biol Chem. 1994. PMID: 7512955
-
Boric acid transport activity of human aquaporins expressed in Xenopus oocytes.Physiol Rep. 2022 Jan;10(1):e15164. doi: 10.14814/phy2.15164. Physiol Rep. 2022. PMID: 35014212 Free PMC article.
-
Regulation, Diversity and Evolution of Boron Transporters in Plants.Plant Cell Physiol. 2021 Sep 24;62(4):590-599. doi: 10.1093/pcp/pcab025. Plant Cell Physiol. 2021. PMID: 33570563 Review.
-
Major intrinsic proteins in biomimetic membranes.Adv Exp Med Biol. 2010;679:127-42. doi: 10.1007/978-1-4419-6315-4_10. Adv Exp Med Biol. 2010. PMID: 20666229 Review.
Cited by
-
The ever expanding role of aquaglyceroporins: confirmation of protein-facilitated boron transport.Plant Signal Behav. 2010 Feb;5(2):132-3. doi: 10.4161/psb.5.2.10305. Epub 2010 Feb 12. Plant Signal Behav. 2010. PMID: 20009511 Free PMC article.
-
Identification of differentially expressed transcripts from leaves of the boron tolerant plant Gypsophila perfoliata L.Plant Cell Rep. 2008 Aug;27(8):1411-22. doi: 10.1007/s00299-008-0560-7. Epub 2008 May 27. Plant Cell Rep. 2008. PMID: 18504585
-
Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species.J Appl Genet. 2017 Nov;58(4):421-435. doi: 10.1007/s13353-017-0403-8. Epub 2017 Aug 4. J Appl Genet. 2017. PMID: 28779288 Free PMC article.
-
Transcription profiles of boron-deficiency-responsive genes in citrus rootstock root by suppression subtractive hybridization and cDNA microarray.Front Plant Sci. 2015 Jan 28;5:795. doi: 10.3389/fpls.2014.00795. eCollection 2014. Front Plant Sci. 2015. PMID: 25674093 Free PMC article.
-
Dynamic changes in the osmotic water permeability of protoplast plasma membrane.Plant Physiol. 2004 Aug;135(4):2301-17. doi: 10.1104/pp.104.043000. Epub 2004 Aug 13. Plant Physiol. 2004. PMID: 15310831 Free PMC article.
References
-
- Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. - PubMed
-
- Agre P, Mathai JC, Smith BL, Preston GM. Functional analyses of aquaporin water channel proteins. Methods Enzymol. 1999;294:550–572. - PubMed
-
- Barone LM, Shih C, Wasserman BP. Mercury-induced conformational changes and identification of conserved surface loops in plasma membrane aquaporins from higher plants: topology of PMIP31 from Beta vulgaris L. J Biol Chem. 1997;272:30672–30677. - PubMed
-
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 1999;18:565–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

