Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca(2+)
- PMID: 11082112
- PMCID: PMC1572423
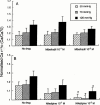
- DOI: 10.1038/sj.bjp.0703650
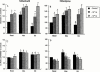
Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca(2+)
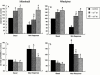
Abstract
1. Ca(2+) entry mechanisms underlying spontaneous arteriolar tone and acute myogenic reactivity remain uncertain. These studies aimed to compare the effects of nifedipine and the putative T-channel blocker, mibefradil, on arteriolar myogenic responsiveness and intracellular Ca(2+) (Ca(2+)(i)). 2. First order cremaster muscle arterioles (1A) were isolated from rats, cannulated, pressurized to 70 mmHg in the absence of intraluminal flow, and mechanical responses studied by video microscopy. The Ca(2+)(i) was measured using fluorescence imaging of Fura 2 loaded arterioles. 3. Both nifedipine and mibefradil showed dose-dependent inhibition of spontaneous myogenic tone (at 70 mmHg; pEC(50) 7.04+/-0.17 vs 6.65+/-0.20 respectively, n=6 for both, n.s.) and KCl-induced vasoconstriction (at 70 mmHg; pEC(50) 6.93+/-0. 38 vs 6.45+/-0.27 respectively, n=6 for both, n.s.). 4. In arterioles maintained at 50 mmHg, nifedipine (10(-7) and 10(-5) M) caused a concentration dependent reduction in Ca(2+)(i), however, mibefradil (10(-7) and 10(-5) M) had no effect. Furthermore nifedipine significantly attenuated the increase in Ca(2+)(i) associated with an acute pressure step (50 - 120 mmHg) whereas mibefradil was considerably less effective. 5. Mibefradil (10(-7) M) significantly attenuated contractile responses to 60 mM KCl without altering the KCl-induced increase in Ca(2+)(i), in contrast to nifedipine (10(-7) M) which reduced both Ca(2+)(i) and contraction. 6. Membrane potential of arterioles with spontaneous myogenic tone (70 mmHg) was -41.5+/-1. 0 mV. Nifedipine (10(-7) or 10(-5) M) had no effect on membrane potential, however mibefradil (10(-5) M) caused significant depolarization. 7. In summary, both mibefradil and nifedipine inhibit arteriolar spontaneous tone and acute myogenic reactivity. While there may be overlap in the mechanisms by which these agents inhibit tone, differences in effects on membrane potential and intracellular Ca(2+) levels suggest mibefradil exhibits actions other than blockade of Ca(2+) entry in skeletal muscle arterioles.
Figures

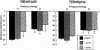
Similar articles
-
Pharmacological evidence for capacitative Ca(2+) entry in cannulated and pressurized skeletal muscle arterioles.Br J Pharmacol. 2001 Sep;134(2):247-56. doi: 10.1038/sj.bjp.0704270. Br J Pharmacol. 2001. PMID: 11564642 Free PMC article.
-
Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling.Am J Physiol Heart Circ Physiol. 2005 Oct;289(4):H1326-34. doi: 10.1152/ajpheart.00323.2005. Epub 2005 Apr 29. Am J Physiol Heart Circ Physiol. 2005. PMID: 15863456
-
Nonuniform changes in arteriolar myogenic tone within skeletal muscle following hindlimb unweighting.J Appl Physiol (1985). 2002 Mar;92(3):1145-51. doi: 10.1152/japplphysiol.01031.2000. J Appl Physiol (1985). 2002. PMID: 11842052
-
Inhibition of vascular myogenic tone and reactivity by calcium antagonists.J Hypertens Suppl. 1989 Sep;7(4):S141-5; discussion S146. J Hypertens Suppl. 1989. PMID: 2681591 Review.
-
Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways.Clin Exp Pharmacol Physiol. 2002 Jul;29(7):612-9. doi: 10.1046/j.1440-1681.2002.03698.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12060106 Review.
Cited by
-
Mechanical control of cation channels in the myogenic response.Am J Physiol Heart Circ Physiol. 2011 Aug;301(2):H331-43. doi: 10.1152/ajpheart.00131.2011. Epub 2011 May 13. Am J Physiol Heart Circ Physiol. 2011. PMID: 21572020 Free PMC article.
-
Atherogenic, fibrotic and glucose utilising actions of glucokinase activators on vascular endothelium and smooth muscle.Cardiovasc Diabetol. 2014 Apr 15;13:80. doi: 10.1186/1475-2840-13-80. Cardiovasc Diabetol. 2014. PMID: 24731772 Free PMC article.
-
Acid-base regulation and sensing: Accelerators and brakes in metabolic regulation of cerebrovascular tone.J Cereb Blood Flow Metab. 2018 Apr;38(4):588-602. doi: 10.1177/0271678X17733868. Epub 2017 Oct 6. J Cereb Blood Flow Metab. 2018. PMID: 28984162 Free PMC article. Review.
-
Low-dose ouabain constricts small arteries from ouabain-hypertensive rats: implications for sustained elevation of vascular resistance.Am J Physiol Heart Circ Physiol. 2009 Sep;297(3):H1140-50. doi: 10.1152/ajpheart.00436.2009. Epub 2009 Jul 17. Am J Physiol Heart Circ Physiol. 2009. PMID: 19617413 Free PMC article.
-
Conducted dilatation to ATP and K+ in rat skeletal muscle arterioles.Acta Physiol (Oxf). 2017 Jan;219(1):202-218. doi: 10.1111/apha.12656. Epub 2016 Feb 22. Acta Physiol (Oxf). 2017. PMID: 26804547 Free PMC article.
References
-
- BEAN B.P. Classes of calcium channels in vertebrate cells. Ann. Rev. Physiol. 1989;51:367–384. - PubMed
-
- CAMPBELL W.B., HARDER D.R. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ. Res. 1999;84:484–488. - PubMed
-
- CHEN X-L., BAYLISS D.A., FERN R.J., BARRETT P.Q. A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+ Am. J. Physiol. 1999;276:F674–F683. - PubMed
-
- CLOZEL J.P., ERTEL E.A., ERTEL S.I. Discovery and main pharmacological properties of mibefradil (Ro 40-5967), the first selective T-type calcium channel blocker. J. Hypertens. 1997;1 Suppl. 5:S17–S25. - PubMed
-
- DAVIS M.J., HILL M.A. Signaling mechanisms underlying the vascular myogenic response. Physiological Revs. 1999;79:387–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

