Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa
- PMID: 11083634
- PMCID: PMC90199
- DOI: 10.1128/AAC.44.12.3317-3321.2000
Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa
Abstract
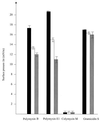
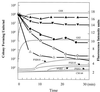
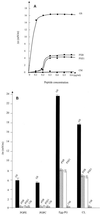
Polymyxins B and E1 and gramicidin S are bacterium-derived cationic antimicrobial peptides. The polymyxins were more potent than gramicidin S against Pseudomonas aeruginosa, with MICs of 0.125 to 0. 25 and 8 microg/ml, respectively. These peptides differed in their affinities for binding to lipopolysaccharide, but all were able to permeabilize the outer membrane of wild-type P. aeruginosa PAO1 strain H103, suggesting differences in their mechanisms of self-promoted uptake. Gramicidin S caused rapid depolarization of the bacterial cytoplasmic membrane at concentrations at which no killing was observed within 30 min, whereas, conversely, the concentrations of the polymyxins that resulted in rapid killing resulted in minimal depolarization. These data indicate that the depolarization of the cytoplasmic membrane by these peptides did not correlate with bacterial cell lethality.
Figures
Similar articles
-
Novel cationic peptide TP359 down-regulates the expression of outer membrane biogenesis genes in Pseudomonas aeruginosa: a potential TP359 anti-microbial mechanism.BMC Microbiol. 2016 Aug 22;16(1):192. doi: 10.1186/s12866-016-0808-2. BMC Microbiol. 2016. PMID: 27549081 Free PMC article.
-
Use of steroids to monitor alterations in the outer membrane of Pseudomonas aeruginosa.J Bacteriol. 1997 Nov;179(22):7004-10. doi: 10.1128/jb.179.22.7004-7010.1997. J Bacteriol. 1997. PMID: 9371446 Free PMC article.
-
The interaction of a recombinant cecropin/melittin hybrid peptide with the outer membrane of Pseudomonas aeruginosa.Mol Microbiol. 1994 Jun;12(6):951-8. doi: 10.1111/j.1365-2958.1994.tb01083.x. Mol Microbiol. 1994. PMID: 7934902
-
Antibiotic uptake pathways across the outer membrane of Pseudomonas aeruginosa.Antibiot Chemother (1971). 1987;39:172-81. doi: 10.1159/000414344. Antibiot Chemother (1971). 1987. PMID: 2823689 Review. No abstract available.
-
Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics.Cell Mol Life Sci. 2009 Dec;66(23):3821-6. doi: 10.1007/s00018-009-0129-9. Epub 2009 Aug 23. Cell Mol Life Sci. 2009. PMID: 19701717 Free PMC article. Review.
Cited by
-
Novel cationic peptide TP359 down-regulates the expression of outer membrane biogenesis genes in Pseudomonas aeruginosa: a potential TP359 anti-microbial mechanism.BMC Microbiol. 2016 Aug 22;16(1):192. doi: 10.1186/s12866-016-0808-2. BMC Microbiol. 2016. PMID: 27549081 Free PMC article.
-
Polymyxin Resistance in Acinetobacter baumannii: Genetic Mutations and Transcriptomic Changes in Response to Clinically Relevant Dosage Regimens.Sci Rep. 2016 May 19;6:26233. doi: 10.1038/srep26233. Sci Rep. 2016. PMID: 27195897 Free PMC article.
-
Investigation of d-Amino Acid-Based Surfactants and Nanocomposites with Gold and Silica Nanoparticles as against Multidrug-Resistant Bacteria Agents.ACS Omega. 2022 Dec 8;7(50):46146-46155. doi: 10.1021/acsomega.2c04220. eCollection 2022 Dec 20. ACS Omega. 2022. PMID: 36570237 Free PMC article.
-
The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation.Appl Environ Microbiol. 2007 Oct;73(20):6620-8. doi: 10.1128/AEM.00881-07. Epub 2007 Aug 24. Appl Environ Microbiol. 2007. PMID: 17720841 Free PMC article.
-
Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance.Biochim Biophys Acta Mol Cell Res. 2023 Mar;1870(3):119407. doi: 10.1016/j.bbamcr.2022.119407. Epub 2022 Dec 18. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36543281 Free PMC article. Review.
References
-
- Dixon R A, Chopra I. Polymyxin B and polymyxin B nonapeptide alter cytoplasmic membrane permeability in Escherichia coli. J Antimicrob Chemother. 1986;18:557–563. - PubMed
-
- Fried V A, Rothfield L I. Interactions between lipopolysaccharide and phosphatidylethanolamine in molecular monolayers. Biochim Biophys Acta. 1978;514:69–82. - PubMed
-
- Izumiya N, Kato T, Aoyaga H, Waki M, Kondo M. Synthetic aspects of biologically active cyclic peptides: gramicidin S and tyrocidines. New York, N.Y: Helsted Press; 1979. pp. 49–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

