Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa
- PMID: 11083634
- PMCID: PMC90199
- DOI: 10.1128/AAC.44.12.3317-3321.2000
Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa
Abstract
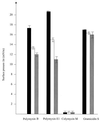
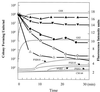
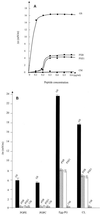
Polymyxins B and E1 and gramicidin S are bacterium-derived cationic antimicrobial peptides. The polymyxins were more potent than gramicidin S against Pseudomonas aeruginosa, with MICs of 0.125 to 0. 25 and 8 microg/ml, respectively. These peptides differed in their affinities for binding to lipopolysaccharide, but all were able to permeabilize the outer membrane of wild-type P. aeruginosa PAO1 strain H103, suggesting differences in their mechanisms of self-promoted uptake. Gramicidin S caused rapid depolarization of the bacterial cytoplasmic membrane at concentrations at which no killing was observed within 30 min, whereas, conversely, the concentrations of the polymyxins that resulted in rapid killing resulted in minimal depolarization. These data indicate that the depolarization of the cytoplasmic membrane by these peptides did not correlate with bacterial cell lethality.
Figures
References
-
- Dixon R A, Chopra I. Polymyxin B and polymyxin B nonapeptide alter cytoplasmic membrane permeability in Escherichia coli. J Antimicrob Chemother. 1986;18:557–563. - PubMed
-
- Fried V A, Rothfield L I. Interactions between lipopolysaccharide and phosphatidylethanolamine in molecular monolayers. Biochim Biophys Acta. 1978;514:69–82. - PubMed
-
- Izumiya N, Kato T, Aoyaga H, Waki M, Kondo M. Synthetic aspects of biologically active cyclic peptides: gramicidin S and tyrocidines. New York, N.Y: Helsted Press; 1979. pp. 49–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

