Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils
- PMID: 11083784
- PMCID: PMC97769
- DOI: 10.1128/IAI.68.12.6697-6703.2000
Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils
Abstract
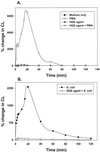
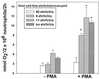
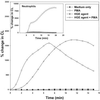
The human granulocytic ehrlichiosis (HGE) agent, which replicates in neutrophils, was found not to induce superoxide anion (O(2)(-)) generation or extracellular release by human peripheral blood neutrophils, as measured by a luminol-dependent chemiluminescence assay or a cytochrome c reduction assay, respectively. Furthermore, the HGE agent completely prevented O(2-) release by neutrophils upon stimulation with phorbol myristate acetate (PMA), formylmethionyl-leucyl-phenylalanine, or Escherichia coli. The inhibition was HGE agent dose dependent, required ehrlichial contact with the host cells, and was reversible upon removal of the extracellular HGE agent bound to the host cells prior to PMA stimulation. Structural integrity of or new protein synthesis by the HGE agent was not required for the inhibition; carbohydrate but not surface protein of the HGE agent was required. The HGE agent did not prevent O(2-) generation in human peripheral blood monocytes derived from the same individual. This neutrophil-specific prevention of O(2-) generation by the HGE agent would be critical in survival of the HGE agent. This is the first demonstration of the rapid inhibition of preexisting NADPH oxidase in human neutrophils by the HGE agent.
Figures



References
-
- Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Baliga B S, Pronczuk A W, Munro H N. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J Biol Chem. 1969;244:4480–4489. - PubMed
-
- Banerjee R, Anguita J, Roos D, Fikrig E. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J Immunol. 2000;164:3946–3949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

