Sequence analysis of TnphoA insertion sites in Vibrio cholerae mutants defective in rugose polysaccharide production
- PMID: 11083805
- PMCID: PMC97790
- DOI: 10.1128/IAI.68.12.6857-6864.2000
Sequence analysis of TnphoA insertion sites in Vibrio cholerae mutants defective in rugose polysaccharide production
Abstract
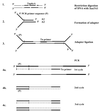
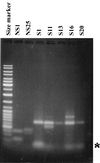
Vibrio cholerae can switch from a smooth to a wrinkled or rugose colony phenotype characterized by the secretion of a polysaccharide that enables the bacteria to survive harsh environmental conditions. In order to understand the genetic basis of rugosity, we isolated TnphoA-induced stable, smooth mutants of two O1 El Tor rugose strains and mapped the insertion sites in several of the mutants using a modified Y-adapter PCR technique. One of the TnphoA insertions was mapped to the first gene of the vps region that was previously shown to encode the rugose polysaccharide biosynthesis cluster. Three insertions were mapped to a previously unknown hlyA-like gene, also in the vps region. Five other insertions were found in loci unlinked to the vps region: (i) in the epsD gene (encodes the "secretin" of the extracellular protein secretion apparatus), (ii) in a hydG-like gene (encodes a sigma(54)-dependent transcriptional activator similar to HydG involved in labile hydrogenase production in Escherichia coli, (iii) in a gene encoding malic acid transport protein upstream of a gene similar to yeiE of E. coli (encodes a protein with similarities to LysR-type transcriptional activators), (iv) in dxr (encodes 1-deoxy-D-xylulose 5-phosphate reductoisomerase), and (v) in the intergenic region of lpd and odp (encode enzymes involved in the pyruvate dehydrogenase complex formation). These data suggest the involvement of a complex regulatory network in rugose polysaccharide production and highlight the general utility of the Y-adapter PCR technique described here for rapid mapping of transposon insertion sites.
Figures


References
-
- Chiang S L, Mekalanos J J. Use of signature tagged transposon mutagenesis to identify Vibrio choleraegenes critical for colonization. Mol Microbiol. 1998;27:797–805. - PubMed
-
- Colwell R R, Brayton P R, Grimes D J, Roszak D B, Huq A, Palmer L M. Viable but non-culturable Vibrio choleraeand related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 1985;3:817–820.
-
- Colwell R R, Huq A. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 117–133.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

