Cellular internalization of cytolethal distending toxin from Haemophilus ducreyi
- PMID: 11083812
- PMCID: PMC97797
- DOI: 10.1128/IAI.68.12.6903-6911.2000
Cellular internalization of cytolethal distending toxin from Haemophilus ducreyi
Abstract
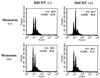
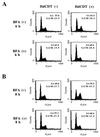
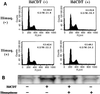
The chancroid bacterium Haemophilus ducreyi produces a toxin (HdCDT) which is a member of the recently discovered family of cytolethal distending toxins (CDTs). These protein toxins prevent the cyclin-dependent kinase cdc2 from being activated, thus blocking the transition of cells from the G(2) phase into mitosis, with the consequent arrest of intoxicated cells in G(2). It is not known whether these toxins act by signaling from the cell surface or intracellularly only. Here we report that HdCDT has to undergo at least internalization before being able to act. Cellular intoxication was inhibited (i) by removal of clathrin coats via K(+) depletion, (ii) by treatment with drugs that inhibit receptor clustering into coated pits, and (iii) in cells genetically manipulated to fail in clathrin-dependent endocytosis. Intoxication was also completely inhibited in cells treated with bafilomycin A1 or nocodazole and in cells incubated at 18 degrees C, i.e., under conditions known to block the fusion of early endosomes with downstream compartments. Moreover, disruption of the Golgi complex by treatment with brefeldin A or ilimaquinone blocked intoxication. In conclusion, our data indicate that HdCDT enters cells via clathrin-coated pits and has to be transported via the Golgi complex in order to intoxicate cells. This is the first member of the family of CDTs for which cellular internalization and some details of the pathway have been demonstrated.
Figures






References
-
- Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. - PubMed
-
- Cieplak W, Jr, Messer R J, Konkel M E, Grant C C R. Role of potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol Microbiol. 1995;16:789–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

