Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii
- PMID: 11083816
- PMCID: PMC97801
- DOI: 10.1128/IAI.68.12.6932-6938.2000
Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii
Abstract
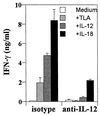
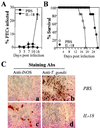
Innate resistance to Toxoplasma gondii is dependent on the ability of interleukin-12 (IL-12) to stimulate natural killer (NK) cell production of gamma interferon (IFN-gamma). Since IL-18 is a potent enhancer of IL-12-induced production of IFN-gamma by NK cells, SCID mice (which lack an adaptive immune response) were used to assess the role of IL-18 in innate resistance to T. gondii. Administration of anti-IL-18 to SCID mice infected with T. gondii resulted in an early reduction in serum levels of IFN-gamma but did not significantly decrease resistance to this infection. In contrast, administration of exogenous IL-18 to infected SCID mice resulted in increased production of IFN-gamma, reduced parasite burden, and a delay in time to death. The protective effects of IL-18 treatment correlated with increased NK cell numbers and cytotoxic activity at the local site of administration and with elevated levels of inducible nitrous oxide synthose in the spleens of treated mice. In addition, in vivo depletion studies demonstrated that the ability of exogenous IL-18 to enhance resistance to T. gondii was dependent on IL-12, IFN-gamma, and NK cells. Together, these studies demonstrate that although endogenous IL-18 appears to have a limited role in innate resistance to T. gondii, treatment with IL-18 can augment NK cell-mediated immunity to this pathogen.
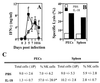
Figures
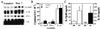
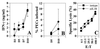


References
-
- Altare F, Durandy A, Lammas D, Emile J-F, Lamhamedi S, Deist F L, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob J A, Meinl E, Segal A W, Fischer A, Kumararatne D, Casanova J-L. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. - PubMed
-
- Bazan J F, Timans J C, Kastelein R. A newly defined interleukin-1? Nature. 1996;379:591–591. - PubMed
-
- Bohn E, Sing A, Zumbihl R, Biefeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. - PubMed
-
- Cai G, Kastelein R A, Hunter C A. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur J Immunol. 1999;29:2658–2665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

