Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes
- PMID: 11083817
- PMCID: PMC97802
- DOI: 10.1128/IAI.68.12.6939-6945.2000
Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes
Abstract
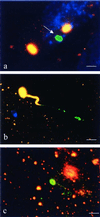
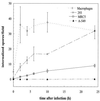
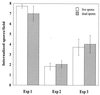
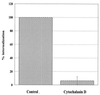
Encephalitozoon cuniculi is an obligate intracellular, spore-forming parasite belonging to the microsporidia that can cause disseminated infection in immunocompromised persons. E. cuniculi spores infect host cells by germination, i.e., by explosively everting the polar filament, through which the spore contents (sporoplasms) are subsequently injected into the cytoplasm. In addition, we observed intracellular, nongerminated spores in various nonprofessional phagocytes. In MRC5 cells, the number of internalized spores was approximately 10-fold higher than the number of injected sporoplasms. Compared to the rate of uptake by human monocyte-derived macrophages, internalization rates by A549 cells, MRC5 cells, and 293 cells were 0.6, 4.4, and 22.2%, respectively. The mechanism of uptake was studied in MRC5 cells. Killed spores were internalized at the same rate as live spores, indicating that nongerminated parasites do not actively participate in cell entry. Cytochalasin D inhibited uptake of spores by 95%, demonstrating an actin-dependent process. By electron and epifluorescence microscopy, intracellular spores were found in a tightly fitting membrane-bound compartment. The vacuole containing the spores was positive for the lysosomal membrane protein LAMP-1 and colocalized with the late endosomal-lysosomal content marker rhodamine dextran. Our results show that, in addition to the unique way in which microsporidia infect cells, E. cuniculi spores enter nonprofessional phagocytes by phagocytosis and traffic into a late endosomal-lysosomal compartment.
Figures


References
-
- Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;7:593–623. - PubMed
-
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97:77–89. - PubMed
-
- Canning E, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986.
-
- Canning E U. Microsporidia. In: Kreier J P, Vaker J R, editors. Parasitic protozoa. 2nd ed. Vol. 6. New York, N.Y: Academic Press; 1993. pp. 299–385.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

