Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation
- PMID: 11083820
- PMCID: PMC97805
- DOI: 10.1128/IAI.68.12.6962-6969.2000
Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation
Abstract
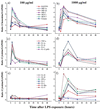
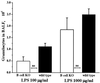
Recruitment of neutrophils to lung tissue and airspaces is a hallmark of inflammatory events following inhalation of endotoxins. We studied the role of different lymphocyte subsets in this inflammation, which is assumed to primarily involve the innate immune system. Inhalation of aerosolized Escherichia coli lipopolysaccharide (LPS) in mice induced a dose-dependent increase in neutrophils in bronchoalveolar lavage fluid, reaching a maximum after 12 h at a low dose and after 24 h at a high dose. Profiles of gene expression in lung tissue indicated an early (2 h) and transient onset of proinflammatory cytokines and chemokines by a low dose of LPS, while a high dose caused more delayed and sustained (6 to 12 h) activation. Gamma interferon, interleukin-2 (IL-2), RANTES, and the alpha chain of the IL-2 receptor were not expressed at a low dose, whereas a high dose of LPS induced a strong expression of these genes, indicating a dose-dependent activation of T cells. A similar pattern was observed for IL-17, supporting a contribution of T cells to the neutrophilic inflammation only at high-dose exposure to LPS. The involvement of lymphocytes in the inflammatory response was further studied using mice with functional deficiencies in defined lymphocyte subsets. Both gammadelta T-cell- and B-cell-deficient mice displayed a response similar to that of the corresponding wild-type strains. Selective depletion of NK cells by in vivo administration of the pk136 antibody did not significantly affect the recruitment of neutrophils into airspaces. Thus, neither NK cells, B cells, nor gammadelta T cells appeared to participate in the host response, suggesting that among the lymphocyte subsets, alphabeta T cells are exclusively involved in endotoxin-induced airway inflammation.
Figures





Similar articles
-
γδ T cells protect against LPS-induced lung injury.J Leukoc Biol. 2016 Feb;99(2):373-86. doi: 10.1189/jlb.4A0115-017RR. Epub 2015 Oct 1. J Leukoc Biol. 2016. PMID: 26428678 Free PMC article.
-
gammadelta T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation.Immunology. 2003 Jan;108(1):98-108. doi: 10.1046/j.1365-2567.2003.01561.x. Immunology. 2003. PMID: 12519308 Free PMC article.
-
IL-17-producing T lymphocytes in lung tissue and in the bronchoalveolar space after exposure to endotoxin from Escherichia coli in vivo--effects of anti-inflammatory pharmacotherapy.Pulm Pharmacol Ther. 2009 Jun;22(3):199-207. doi: 10.1016/j.pupt.2008.12.005. Epub 2008 Dec 24. Pulm Pharmacol Ther. 2009. PMID: 19121406
-
IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger.J Immunol. 2003 Feb 15;170(4):2106-12. doi: 10.4049/jimmunol.170.4.2106. J Immunol. 2003. PMID: 12574382
-
Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice.J Exp Med. 1999 Feb 1;189(3):553-62. doi: 10.1084/jem.189.3.553. J Exp Med. 1999. PMID: 9927517 Free PMC article.
Cited by
-
γδT cells but not αβT cells contribute to sepsis-induced white matter injury and motor abnormalities in mice.J Neuroinflammation. 2017 Dec 20;14(1):255. doi: 10.1186/s12974-017-1029-9. J Neuroinflammation. 2017. PMID: 29262837 Free PMC article.
-
Electrical vagus nerve stimulation is a promising approach to reducing pulmonary complications after an esophagectomy: an experimental rodent model.Immunol Res. 2024 Dec;72(6):1247-1258. doi: 10.1007/s12026-024-09523-3. Epub 2024 Jul 31. Immunol Res. 2024. PMID: 39083131 Free PMC article.
-
High protein diet increases the risk of allergic sensitization but not asthma in mice through modulation of the cytokine milieu toward Th2 bias.World Allergy Organ J. 2025 Feb 6;18(2):101031. doi: 10.1016/j.waojou.2025.101031. eCollection 2025 Feb. World Allergy Organ J. 2025. PMID: 39995506 Free PMC article.
-
Poly-L-Arginine Acts Synergistically with LPS to Promote the Release of IL-6 and IL-8 via p38/ERK Signaling Pathways in NCI-H292 Cells.Inflammation. 2016 Feb;39(1):47-53. doi: 10.1007/s10753-015-0221-2. Inflammation. 2016. PMID: 26246181
-
One-hit, two-hit . . . is there really any benefit?Clin Exp Immunol. 2005 Aug;141(2):211-4. doi: 10.1111/j.1365-2249.2005.02853.x. Clin Exp Immunol. 2005. PMID: 15996184 Free PMC article. No abstract available.
References
-
- Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. - PubMed
-
- Allen R D, Staley T A, Sidman C L. Differential cytokine expression in acute and chronic graft-versus-host-disease. Eur J Immunol. 1993;23:333–337. - PubMed
-
- Berg J T, Lee S T, Thepen T, Lee C Y, Tsan M F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–2819. - PubMed
-
- Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol. 1996;15:64–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

