Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages
- PMID: 11083824
- PMCID: PMC97809
- DOI: 10.1128/IAI.68.12.6997-7002.2000
Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages
Abstract
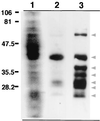
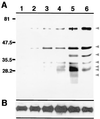
Considerable effort has focused on the identification of proteins secreted from Mycobacterium spp. that contribute to the development of protective immunity. Little is known, however, about the release of mycobacterial proteins from the bacterial phagosome and the potential role of these molecules in chronically infected macrophages. In the present study, the release of mycobacterial surface proteins from the bacterial phagosome into subcellular compartments of infected macrophages was analyzed. Mycobacterium bovis BCG was surface labeled with fluorescein-tagged succinimidyl ester, an amine-reactive probe. The fluorescein tag was then used as a marker for the release of bacterial proteins in infected macrophages. Fractionation studies revealed bacterial proteins within subcellular compartments distinct from mycobacteria and mycobacterial phagosomes. To identify these proteins, subcellular fractions free of bacteria were probed with mycobacterium-specific antibodies. The fibronectin attachment protein and proteins of the antigen 85-kDa complex were identified among the mycobacterial proteins released from the bacterial phagosome.
Figures



References
-
- Abou-Zeid C, Smith I, Grange J M, Ratliff T L, Steele J, Rook G A. The secreted antigens of Mycobacterium tuberculosisand their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988;134:531–538. - PubMed
-
- Beatty W L, Rhoades E R, Ullrich H-J, Chatterjee D, Heuser J E, Russell D G. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

