Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth
- PMID: 11083830
- PMCID: PMC97815
- DOI: 10.1128/IAI.68.12.7049-7060.2000
Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth
Abstract
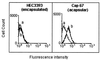
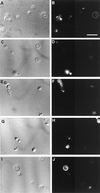
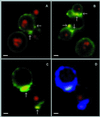
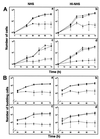
A major ceramide monohexoside (CMH) was purified from lipidic extracts of Cryptococcus neoformans. This molecule was analyzed by high-performance thin-layer chromatography (HPTLC), gas chromatography coupled with mass spectrometry, and fast atom bombardment-mass spectrometry. The cryptococcal CMH is a beta-glucosylceramide, with the carbohydrate residue attached to 9-methyl-4,8-sphingadienine in amidic linkage to 2-hydroxyoctadecanoic acid. Sera from patients with cryptococcosis and a few other mycoses reacted with the cryptococcal CMH. Specific antibodies were purified from patients' sera by immunoadsorption on the purified glycolipid followed by protein G affinity chromatography. The purified antibodies to CMH (mainly immunoglobulin G1) bound to different strains and serological types of C. neoformans, as shown by flow cytofluorimetry and immunofluorescence labeling. Transmission electron microscopy of yeasts labeled with immunogold-antibodies to CMH and immunostaining of isolated cell wall lipid extracts separated by HPTLC showed that the cryptococcal CMH predominantly localizes to the fungal cell wall. Confocal microscopy revealed that the beta-glucosylceramide accumulates mostly at the budding sites of dividing cells with a more disperse distribution at the cell surface of nondividing cells. The increased density of sphingolipid molecules seems to correlate with thickening of the cell wall, hence with its biosynthesis. The addition of human antibodies to CMH to cryptococcal cultures of both acapsular and encapsulated strains of C. neoformans inhibited cell budding and cell growth. This process was complement-independent and reversible upon removal of the antibodies. The present data suggest that the cryptococcal beta-glucosylceramide is a fungal antigen that plays a role on the cell wall synthesis and yeast budding and that antibodies raised against this component are inhibitory in vitro.
Figures






References
-
- Barreto-Bergter E, Vermelho A B, Hartmann R, Pohlentz G, Klein R A, Egge H. Structural characterization of neutral glycosphingolipids from Trypanosoma cruzi. Mol Biochem Parasitol. 1992;51:263–270. - PubMed
-
- Barreto-Bergter E, Travassos L R, Gorin P A J. Chemical structure of the d-galacto-d-mannan component from hyphae of Aspergillus niger and other Aspergillus spp. Carbohydr Res. 1980;86:273–285.
-
- Bielawska A, Linardic C M, Hannun Y A. Modulation of cell growth and differentiation by ceramide. FEBS Lett. 1992;307:211–214. - PubMed
-
- Chen S C A, Muller M, Zhou J Z, Wright L C, Sorrel T C. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175:414–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

