OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival
- PMID: 11083832
- PMCID: PMC97817
- DOI: 10.1128/IAI.68.12.7069-7077.2000
OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival
Abstract
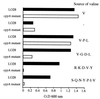
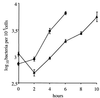
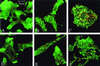
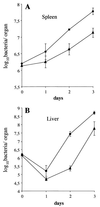
We identified a new oligopeptide permease operon in the pathogen Listeria monocytogenes. This opp operon consists of five genes (oppA, oppB, oppC, oppD, and oppF) and displays the same genetic organization as those of several bacterial species. The first gene of this operon, oppA, encodes a 62-kDa protein sharing 33% identity with OppA of Bacillus subtilis and is expressed predominantly during exponential growth. The function of oppA was studied by constructing an oppA deletion mutant. The phenotype analysis of this mutant revealed that OppA mediates the transport of oligopeptides and is required for bacterial growth at low temperature. The wild-type phenotype was restored by complementing the mutant with oppA. We also found that OppA is involved in intracellular survival in macrophages and in bacterial growth in organs of mice infected with L. monocytogenes, although the level of virulence was not altered in the mutant. These results show the major role of OppA in the uptake of oligopeptides and the pleiotropic effects of this oligopeptide-binding protein on the behavior of this pathogen in the environment and in its host.
Figures




References
-
- Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;l41:41–49. - PubMed
-
- Bayles D O, Wilkinson B J. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett Appl Microbiol. 2000;30:23–27. - PubMed
-
- Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–117. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

