Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex
- PMID: 11085745
- PMCID: PMC2193184
- DOI: 10.1084/jem.192.10.1425
Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex
Abstract
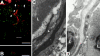
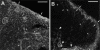
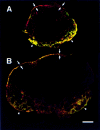
Lymph-borne, soluble factors (e.g., chemokines and others) influence lymphocyte recirculation and endothelial phenotype at high endothelial venules (HEVs) in lymph node cortex. Yet the route lymph-borne soluble molecules travel from the subcapsular sinus to the HEVs is unclear. Therefore, we injected subcutaneously into mice and rats a wide variety of fluorophore-labeled, soluble molecules and examined their distribution in the draining lymph nodes. Rather than percolating throughout the draining lymph node, all molecules, including microbial lipopolysaccharide, were very visible in the subcapsular and medullary sinuses but were largely excluded from the cortical lymphocyte microenvironments. Exclusion prevailed even during the acute lymph node enlargement accompanying viral infection. However, low molecular mass (MW) molecules, including chemokines, did gain entry into the cortex, but in a very defined manner. Low MW, fluorophore-labeled molecules highlighted the subcapsular sinus, the reticular fibers, and the abluminal and luminal surfaces of the associated HEVs. These low MW molecules were in the fibers of the reticular network, a meshwork of collagen fibers ensheathed by fibroblastic reticular cells that connects the subcapsular sinus floor and the HEVs by intertwining with their basement membranes. Thus, low MW, lymph-borne molecules, including chemokines, traveled rapidly from the subcapsular sinus to the HEVs using the reticular network as a conduit.
Figures




References
-
- Gowans J.L., Knight E.J. The route of re-circulation of lymphocytes in rat. Proc. R. Soc. Lond. Ser. B. 1964;159:257–282. - PubMed
-
- Butcher E.C. Leukocyte-endothelial cell recognitionthree (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
-
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol. Today. 1992;13:106–112. - PubMed
-
- Tanaka Y., Adams D.H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

