Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution
- PMID: 11086008
- PMCID: PMC2174347
- DOI: 10.1083/jcb.151.5.1067
Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution
Abstract
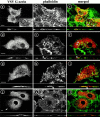
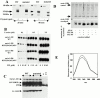
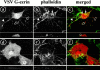
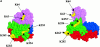
The cytoskeleton-membrane linker protein ezrin has been shown to associate with phosphatidyl-inositol 4,5-bisphosphate (PIP(2))-containing liposomes via its NH(2)-terminal domain. Using internal deletions and COOH-terminal truncations, determinants of PIP(2) binding were located to amino acids 12-115 and 233-310. Both regions contain a KK(X)(n)K/RK motif conserved in the ezrin/radixin/moesin family. K/N mutations of residues 253 and 254 or 262 and 263 did not affect cosedimentation of ezrin 1-333 with PIP(2)-containing liposomes, but their combination almost completely abolished the capacity for interaction. Similarly, double mutation of Lys 63, 64 to Asn only partially reduced lipid interaction, but combined with the double mutation K253N, K254N, the interaction of PIP(2) with ezrin 1-333 was strongly inhibited. Similar data were obtained with full-length ezrin. When residues 253, 254, 262, and 263 were mutated in full-length ezrin, the in vitro interaction with the cytoplasmic tail of CD44 was not impaired but was no longer PIP(2) dependent. This construct was also expressed in COS1 and A431 cells. Unlike wild-type ezrin, it was not any more localized to dorsal actin-rich structures, but redistributed to the cytoplasm without strongly affecting the actin-rich structures. We have thus identified determinants of the PIP(2) binding site in ezrin whose mutagenesis correlates with an altered cellular localization.
Figures






References
-
- Amieva M.R., Litman P., Huang L., Ichimaru E., Furthmayr H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrinin vivo imaging with GFP fusion proteins. J. Cell Sci. 1999;112:111–125. - PubMed
-
- Andréoli C., Martin M., Leborgne R., Reggio H., Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci. 1994;107:2509–2521. - PubMed
-
- Blomberg N., Nilges M. Functional diversity of PH domainsan exhaustive modelling study. Fold. Des. 1997;2:343–355. - PubMed
-
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

