Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change
- PMID: 11086009
- PMCID: PMC2174365
- DOI: 10.1083/jcb.151.5.1081
Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change
Abstract
Conventional kinesin, a dimeric molecular motor, uses ATP-dependent conformational changes to move unidirectionally along a row of tubulin subunits on a microtubule. Two models have been advanced for the major structural change underlying kinesin motility: the first involves an unzippering/zippering of a small peptide (neck linker) from the motor catalytic core and the second proposes an unwinding/rewinding of the adjacent coiled-coil (neck coiled-coil). Here, we have tested these models using disulfide cross-linking of cysteines engineered into recombinant kinesin motors. When the neck linker motion was prevented by cross-linking, kinesin ceased unidirectional movement and only showed brief one-dimensional diffusion along microtubules. Motility fully recovered upon adding reducing agents to reverse the cross-link. When the neck linker motion was partially restrained, single kinesin motors showed biased diffusion towards the microtubule plus end but could not move effectively against a load imposed by an optical trap. Thus, partial movement of the neck linker suffices for directionality but not for normal processivity or force generation. In contrast, preventing neck coiled-coil unwinding by disulfide cross-linking had relatively little effect on motor activity, although the average run length of single kinesin molecules decreased by 30-50%. These studies indicate that conformational changes in the neck linker, not in the neck coiled-coil, drive processive movement by the kinesin motor.
Figures
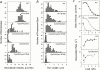
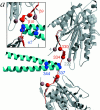

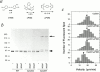
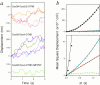


References
-
- Block S.M. Kinesinwhat gives? Cell. 1998;93:5–8. - PubMed
-
- Block S.M., Goldstein L.S.B., Schnapp B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. - PubMed
-
- Case R.B., Pierce D.W., Hom-Booher N., Hart C.L., Vale R.D. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell. 1997;90:959–966. - PubMed
-
- Case R.B., Rice S., Hart C.L., Ly B., Vale R.D. Role of the kinesin neck linker and catalytic core in microtubule-based motility. Curr. Biol. 2000;10:157–160. - PubMed
-
- Chandrasekhar S. Stochastic problems in physics and astronomy. Rev. Mod. Phys. 1943;15:1–89.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

