Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones
- PMID: 11086023
- PMCID: PMC381440
- DOI: 10.1172/JCI11245
Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones
Abstract
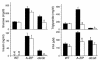
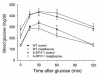
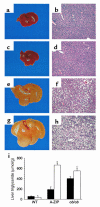
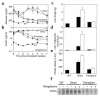
There is uncertainty about the site(s) of action of the antidiabetic thiazolidinediones (TZDs). These drugs are agonist ligands of the transcription factor PPAR gamma, which is abundant in adipose tissue but is normally present at very low levels in liver and muscle. We have studied the effects of TZDs in A-ZIP/F-1 mice, which lack white adipose tissue. The A-ZIP/F-1 phenotype strikingly resembles that of humans with severe lipoatrophic diabetes, including the lack of fat, marked insulin resistance and hyperglycemia, hyperlipidemia, and fatty liver. Rosiglitazone or troglitazone treatment did not reduce glucose or insulin levels, suggesting that white adipose tissue is required for the antidiabetic effects of TZDs. However, TZD treatment was effective in lowering circulating triglycerides and increasing whole body fatty acid oxidation in the A-ZIP/F-1 mice, indicating that this effect occurs via targets other than white adipose tissue. A-ZIP/F-1 mice have markedly increased liver PPAR gamma mRNA levels, which may be a general property of fatty livers. Rosiglitazone treatment increased the triglyceride content of the steatotic livers of A-ZIP/F-1 and ob/ob mice, but not the "lean" livers of fat-transplanted A-ZIP/F-1 mice. In light of this evidence that rosiglitazone acts differently in steatotic livers, the effects of rosiglitazone, particularly on hepatic triglyceride levels, should be examined in humans with hepatic steatosis.
Figures
Comment in
-
Unraveling the mechanism of action of thiazolidinediones.J Clin Invest. 2000 Dec;106(11):1305-7. doi: 10.1172/JCI11705. J Clin Invest. 2000. PMID: 11104782 Free PMC article. No abstract available.
References
-
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. - PubMed
-
- Gavrilova O, Marcus-Samuels B, Leon LR, Vinson C, Reitman ML. Leptin and diabetes in lipoatrophic mice. Nature. 2000;403:850; discussion 850–851. - PubMed
-
- Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

