Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha
- PMID: 11086024
- PMCID: PMC381439
- DOI: 10.1172/JCI11066
Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha
Abstract
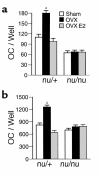
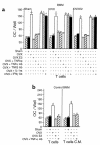
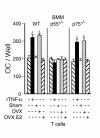
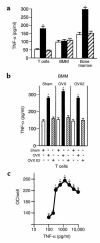
Estrogen deficiency induces bone loss by upregulating osteoclastogenesis by mechanisms not completely defined. We found that ovariectomy-enhanced T-cell production of TNF-alpha, which, acting through the TNF-alpha receptor p55, augments macrophage colony-stimulating factor-induced (M-CSF-induced) and RANKL-induced osteoclastogenesis. Ovariectomy failed to induce bone loss, stimulate bone resorption, or increase M-CSF- and RANKL-dependent osteoclastogenesis in T-cell deficient mice, establishing T cells as essential mediators of the bone-wasting effects of estrogen deficiency in vivo. These findings demonstrate that the ability of estrogen to target T cells, suppressing their production of TNF-alpha, is a key mechanism by which estrogen prevents osteoclastic bone resorption and bone loss.
Figures
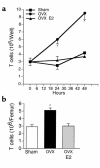
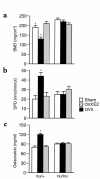
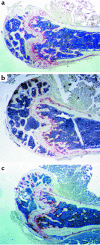
Comment in
-
The mechanisms of estrogen regulation of bone resorption.J Clin Invest. 2000 Nov;106(10):1203-4. doi: 10.1172/JCI11468. J Clin Invest. 2000. PMID: 11086020 Free PMC article. Review. No abstract available.
References
-
- Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. - PubMed
-
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. N Engl J Med. 1995;332:305–311. - PubMed
-
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

