Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis
- PMID: 11086028
- PMCID: PMC381438
- DOI: 10.1172/JCI10793
Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis
Abstract
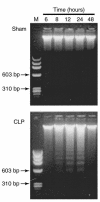
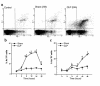
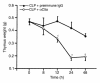
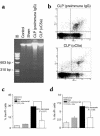
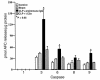
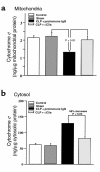
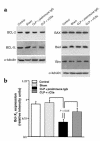
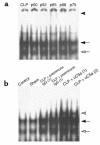
Multiorgan apoptosis occurs during sepsis. Following cecal ligation and puncture (CLP) in rats, thymocytes underwent apoptosis in a time-dependent manner. C5a blockade dramatically reduced thymocyte apoptosis as measured by thymic weight, binding of annexin V to thymocytes, and laddering of thymocyte DNA. When C5a was generated in vivo by infusion of purified cobra venom factor (CVF), thymocyte apoptosis was significantly increased. Similar results were found when CVF was injected in vivo during the early stages of CLP. In animals 12 hours after induction of CLP, there was an increase in the activities of caspase-3, -6, and -9, but not caspase-1 and -8. Cytosolic cytochrome c levels increased by twofold, whereas mitochondrial levels showed a 50% decrease. Western blot analysis revealed that the content of Bcl-X(L) (but not of Bcl-2, BAX, Bad, and Bim) significantly decreased in thymocytes after CLP. C5a blockade in the sepsis model almost completely inhibited caspase-3, -6, and -9 activation, significantly preserved cytochrome c in the mitochondrial fraction, and restored Bcl-X(L) expression. These data suggest that systemic activation of complement induces C5a-dependent apoptosis of thymocytes and that the blockade of C5a during sepsis rescues thymocytes from apoptosis.
Figures

References
-
- Darville T, Giroir B, Jacobs R. The systemic inflammatory response syndrome (SIRS): immunology and potential immunotherapy. Infection. 1993;21:279–290. - PubMed
-
- Papathanassoglou ED, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit Care Med. 2000;28:537–549. - PubMed
-
- Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6:S27–S38. - PubMed
-
- Mahidhara R, Billiar TR. Apoptosis in sepsis. Crit Care Med. 2000;28:N105–N113. - PubMed
-
- Hotchkiss RS, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

