Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate
- PMID: 11086029
- PMCID: PMC381432
- DOI: 10.1172/JCI7236
Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate
Abstract
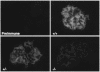
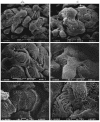
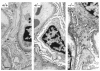
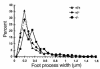
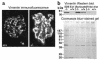
Glomerular epithelial protein 1 (GLEPP1) is a receptor tyrosine phosphatase present on the apical cell surface of the glomerular podocyte. The GLEPP1 gene (PTPRO:) was disrupted at an exon coding for the NH(2)-terminal region by gene targeting in embryonic stem cells. Heterozygote mating produced the expected genotypic ratio of 1:2:1, indicating that the Ptpro(-/-) genotype does not lead to embryonic or neonatal lethality. Kidney and glomerular structure was normal at the gross and light microscopic levels. Scanning and transmission electron microscopy showed that Ptpro(-/-) mice had an amoeboid rather than the typical octopoid structure seen in the wild-type mouse podocyte and that there were blunting and widening of the minor (foot) processes in association with altered distribution of the podocyte intermediate cytoskeletal protein vimentin. Reduced filtration surface area in association with these structural changes was confirmed by finding reduced glomerular nephrin content and reduced glomerular filtration rate in Ptpro(-/-) mice. There was no detectable increase in the urine albumin excretion of Ptpro(-/-) mice. After removal of one or more kidneys, Ptpro(-/-) mice had higher blood pressure than did their wild-type littermates. These data support the conclusion that the GLEPP1 (Ptpro) receptor plays a role in regulating the glomerular pressure/filtration rate relationship through an effect on podocyte structure and function.
Figures


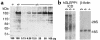
References
-
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat and man. Lab Invest. 1988;59:673–682. - PubMed
-
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. - PubMed
-
- Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E. In vivo enzymatic removal of the α2→6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest. 1996;74:907–920. - PubMed
-
- Kreidberg JA, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

