The proinflammatory mediator macrophage migration inhibitory factor induces glucose catabolism in muscle
- PMID: 11086030
- PMCID: PMC381433
- DOI: 10.1172/JCI9900
The proinflammatory mediator macrophage migration inhibitory factor induces glucose catabolism in muscle
Abstract
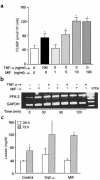
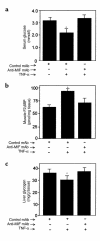
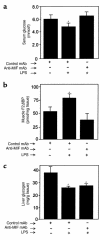
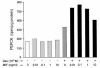
Severe infection or tissue invasion can provoke a catabolic response, leading to severe metabolic derangement, cachexia, and even death. Macrophage migration inhibitory factor (MIF) is an important regulator of the host response to infection. Released by various immune cells and by the anterior pituitary gland, MIF plays a critical role in the systemic inflammatory response by counterregulating the inhibitory effect of glucocorticoids on immune-cell activation and proinflammatory cytokine production. We describe herein an unexpected role for MIF in the regulation of glycolysis. The addition of MIF to differentiated L6 rat myotubes increased synthesis of fructose 2,6-bisphosphate (F2,6BP), a positive allosteric regulator of glycolysis. Increased expression of the enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2) enhanced F2,6BP production and, consequently, cellular lactate production. The catabolic effect of TNF-alpha on myotubes was mediated by MIF, which served as an autocrine stimulus for F2, 6BP production. TNF-alpha administered to mice decreased serum glucose levels and increased muscle F2,6BP levels; pretreatment with a neutralizing anti-MIF mAb completely inhibited these effects. Anti-MIF also prevented hypoglycemia and increased muscle F2,6BP levels in TNF-alpha-knockout mice that were administered LPS, supporting the intrinsic contribution of MIF to these inflammation-induced metabolic changes. Taken together with the recent finding that MIF is a positive, autocrine stimulator of insulin release, these data suggest an important role for MIF in the control of host glucose disposal and carbohydrate metabolism.
Figures

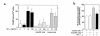

References
-
- Michie HR. Metabolism of sepsis and multiple organ failure. World J Surg. 1996;20:460–464. - PubMed
-
- Rousseau GG, Hue L. Mammalian 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a bifunctional enzyme that controls glycolysis. Prog Nucleic Acid Res Mol Biol. 1993;45:99–127. - PubMed
-
- Furnsinn CS, et al. Acute and chronic exposure to tumor necrosis factor-α fails to affect insulin-stimulated glucose metabolism of isolated rat soleus muscle. Endocrinology. 1997;138:2674–2679. - PubMed
-
- De Blaauw I, et al. TNFα has no direct in vivo metabolic effect on human muscle. Int J Cancer. 1997;71:148–154. - PubMed
-
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor-α: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

