Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating
- PMID: 11087812
- PMCID: PMC17680
- DOI: 10.1073/pnas.220389697
Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating
Abstract
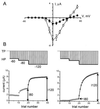
Syntaxin is a key presynaptic protein that binds to N- and P/Q-type Ca(2+) channels in biochemical studies and affects gating of these Ca(2+) channels in expression systems and in synaptosomes. The present study was aimed at understanding the molecular basis of syntaxin modulation of N-type channel gating. Mutagenesis of either syntaxin 1A or the pore-forming alpha(1B) subunit of N-type Ca(2+) channels was combined with functional assays of N-type channel gating in a Xenopus oocyte coexpression system and in biochemical binding experiments in vitro. Our analysis showed that the transmembrane region of syntaxin and a short region within the H3 helical cytoplasmic domain of syntaxin, containing residues Ala-240 and Val-244, appeared critical for the channel modulation but not for biochemical association with the "synprint site" in the II/III loop of alpha(1B). These results suggest that syntaxin and the alpha(1B) subunit engage in two kinds of interactions: an anchoring interaction via the II/III loop synprint site and a modulatory interaction via another site located elsewhere in the channel sequence. The segment of syntaxin H3 found to be involved in the modulatory interaction would lie hidden within the four-helix structure of the SNARE complex, supporting the hypothesis that syntaxin's ability to regulate N-type Ca(2+) channels would be enabled after SNARE complex disassembly after synaptic vesicle exocytosis.
Figures




References
-
- Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. - PubMed
-
- Yoshida A, Oho C, Omori A, Kuwahara R, Ito T, Takahashi M. J Biol Chem. 1992;267:24925–24928. - PubMed
-
- Sollner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. - PubMed
-
- Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. - PubMed
-
- Kee Y, Lin R C, Hsu S C, Scheller R H. Neuron. 1995;14:991–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

