Observation of the noncovalent assembly and disassembly pathways of the chaperone complex MtGimC by mass spectrometry
- PMID: 11087821
- PMCID: PMC18886
- DOI: 10.1073/pnas.240326597
Observation of the noncovalent assembly and disassembly pathways of the chaperone complex MtGimC by mass spectrometry
Abstract
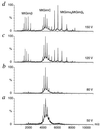
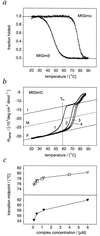
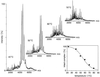
We have analyzed a newly described archaeal GimC/prefoldin homologue, termed MtGimC, by using nanoflow electrospray coupled with time-of-flight MS. The molecular weight of the complex from Methanobacterium thermoautotrophicum corresponds to a well-defined hexamer of two alpha subunits and four beta subunits. Dissociation of the complex within the gas phase reveals a quaternary arrangement of two central subunits, both alpha, and four peripheral beta subunits. By constructing a thermally controlled nanoflow device, we have monitored the thermal stability of the complex by MS. The results of these experiments demonstrate that a significant proportion of the MtGimC hexamer remains intact under low-salt conditions at elevated temperatures. This finding is supported by data from CD spectroscopy, which show that at physiological salt concentrations, the complex remains stable at temperatures above 65 degrees C. Mass spectrometric methods were developed to monitor in real time the assembly of the MtGimC hexamer from its component subunits. By using this methodology, the mass spectra recorded throughout the time course of the experiment showed the absence of any significantly populated intermediates, demonstrating that the assembly process is highly cooperative. Taken together, these data show that the complex is stable under the elevated temperatures that are appropriate for its hyperthermophile host and demonstrate that the assembly pathway leads exclusively to the hexamer, which is likely to be a structural unit in vivo.
Figures


Comment in
-
Mass spectrometry of proteins of known mass.Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14025-7. doi: 10.1073/pnas.011526498. Proc Natl Acad Sci U S A. 2000. PMID: 11114170 Free PMC article. Review. No abstract available.
References
-
- Vainberg I E, Lewis S A, Rommelaere H, Ampe C, Vandekerckhove J, Klein H L, Cowan N J. Cell. 1998;93:863–873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

