Zinc plays a key role in human and bacterial GTP cyclohydrolase I
- PMID: 11087827
- PMCID: PMC17616
- DOI: 10.1073/pnas.240463497
Zinc plays a key role in human and bacterial GTP cyclohydrolase I
Abstract
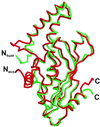
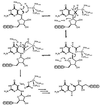
The crystal structure of recombinant human GTP cyclohydrolase I was solved by Patterson search methods by using the coordinates of the Escherichia coli enzyme as a model. The human as well as bacterial enzyme were shown to contain an essential zinc ion coordinated to a His side chain and two thiol groups in each active site of the homodecameric enzymes that had escaped detection during earlier studies of the E. coli enzyme. The zinc ion is proposed to generate a hydroxyl nucleophile for attack of imidazole ring carbon atom eight of the substrate, GTP. It may also be involved in the hydrolytic release of formate from the intermediate, 2-amino-5-formylamino-6-ribosylamino-4(3H)-pyrimidinone 5'-triphosphate, and in the consecutive Amadori rearrangement of the ribosyl moiety.
Figures

References
-
- Brown G M, Williamson J M. In: Escherichia coli and Salmonella typhimurium. Neidhardt F C, editor. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1987. pp. 521–538.
-
- Kaufman S. Annu Rev Nutr. 1993;13:261–286. - PubMed
-
- Ozelius L J, Breakefield X O. Nat Genet. 1994;8:207–209. - PubMed
-
- Yoneyama T, Hatakeyama K. J Biol Chem. 1998;273:20102–20108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

