Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy
- PMID: 11087833
- PMCID: PMC17659
- DOI: 10.1073/pnas.250379497
Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy
Abstract
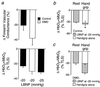
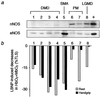
Duchenne muscular dystrophy (DMD) is a fatal disease caused by mutation of the gene encoding the cytoskeletal protein dystrophin. Despite a wealth of recent information about the molecular basis of DMD, effective treatment for this disease does not exist because the mechanism by which dystrophin deficiency produces the clinical phenotype is unknown. In both mouse and human skeletal muscle, dystrophin deficiency results in loss of neuronal nitric oxide synthase, which normally is localized to the sarcolemma as part of the dystrophin-glycoprotein complex. Recent studies in mice suggest that skeletal muscle-derived nitric oxide may play a key role in the regulation of blood flow within exercising skeletal muscle by blunting the vasoconstrictor response to alpha-adrenergic receptor activation. Here we report that this protective mechanism is defective in children with DMD, because the vasoconstrictor response (measured as a decrease in muscle oxygenation) to reflex sympathetic activation was not blunted during exercise of the dystrophic muscles. In contrast, this protective mechanism is intact in healthy children and those with polymyositis or limb-girdle muscular dystrophy, muscle diseases that do not result in loss of neuronal nitric oxide synthase. This clinical investigation suggests that unopposed sympathetic vasoconstriction in exercising human skeletal muscle may constitute a heretofore unappreciated vascular mechanism contributing to the pathogenesis of DMD.
Figures



Comment in
-
The dystrophin-associated glycoprotein complex: what parts can you do without?Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13464-6. doi: 10.1073/pnas.011510597. Proc Natl Acad Sci U S A. 2000. PMID: 11095702 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

