The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters
- PMID: 11087855
- PMCID: PMC27168
- DOI: 10.1073/pnas.97.24.13003
The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters
Abstract
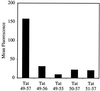
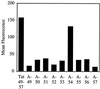
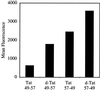
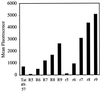
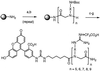
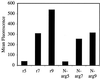
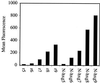
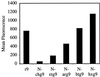
Certain proteins contain subunits that enable their active translocation across the plasma membrane into cells. In the specific case of HIV-1, this subunit is the basic domain Tat(49-57) (RKKRRQRRR). To establish the optimal structural requirements for this translocation process, and thereby to develop improved molecular transporters that could deliver agents into cells, a series of analogues of Tat(49-57) were prepared and their cellular uptake into Jurkat cells was determined by flow cytometry. All truncated and alanine-substituted analogues exhibited diminished cellular uptake, suggesting that the cationic residues of Tat(49-57) play a principal role in its uptake. Charge alone, however, is insufficient for transport as oligomers of several cationic amino acids (histidine, lysine, and ornithine) are less effective than Tat(49-57) in cellular uptake. In contrast, a 9-mer of l-arginine (R9) was 20-fold more efficient than Tat(49-57) at cellular uptake as determined by Michaelis-Menton kinetic analysis. The d-arginine oligomer (r9) exhibited an even greater uptake rate enhancement (>100-fold). Collectively, these studies suggest that the guanidinium groups of Tat(49-57) play a greater role in facilitating cellular uptake than either charge or backbone structure. Based on this analysis, we designed and synthesized a class of polyguanidine peptoid derivatives. Remarkably, the subset of peptoid analogues containing a six-methylene spacer between the guanidine head group and backbone (N-hxg), exhibited significantly enhanced cellular uptake compared to Tat(49-57) and even to r9. Overall, a transporter has been developed that is superior to Tat(49-57), protease resistant, and more readily and economically prepared.
Figures

References
-
- Terwogt J M M, Nuijen B, Huinink W W T B, Beignen J H. Cancer Treat Rev. 1997;23:87–95. - PubMed
-
- Rait A, Pirollo K, Will D W, Peyman A, Rait V, Uhlmann E, Chang E H. Bioconjugate Chem. 2000;11:153–160. - PubMed
-
- Rui Y J, Wang S, Low P S, Thompson D H. J Am Chem Soc. 1998;120:11213–11218.
-
- Kono K, Liu M, Fréchet J M J. Bioconjugate Chem. 1999;10:1115–1121. - PubMed
-
- Ghosh A, Ghosh M, Niu C, Malouin F, Moellmann U, Miller M J. Chem Biol. 1996;3:1011–1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

