Dynamics and folding of single two-stranded coiled-coil peptides studied by fluorescent energy transfer confocal microscopy
- PMID: 11087856
- PMCID: PMC27171
- DOI: 10.1073/pnas.97.24.13021
Dynamics and folding of single two-stranded coiled-coil peptides studied by fluorescent energy transfer confocal microscopy
Abstract
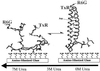
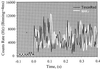
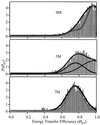
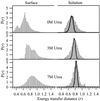
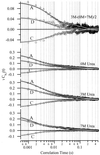
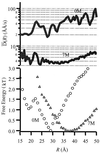
We report single-molecule measurements on the folding and unfolding conformational equilibrium distributions and dynamics of a disulfide crosslinked version of the two-stranded coiled coil from GCN4. The peptide has a fluorescent donor and acceptor at the N termini of its two chains and a Cys disulfide near its C terminus. Thus, folding brings the two N termini of the two chains close together, resulting in an enhancement of fluorescent resonant energy transfer. End-to-end distance distributions have thus been characterized under conditions where the peptide is nearly fully folded (0 M urea), unfolded (7.4 M urea), and in dynamic exchange between folded and unfolded states (3.0 M urea). The distributions have been compared for the peptide freely diffusing in solution and deposited onto aminopropyl silanized glass. As the urea concentration is increased, the mean end-to-end distance shifts to longer distances both in free solution and on the modified surface. The widths of these distributions indicate that the molecules are undergoing millisecond conformational fluctuations. Under all three conditions, these fluctuations gave nonexponential correlations on 1- to 100-ms time scale. A component of the correlation decay that was sensitive to the concentration of urea corresponded to that measured by bulk relaxation kinetics. The trajectories provided effective intramolecular diffusion coefficients as a function of the end-to-end distances for the folded and unfolded states. Single-molecule folding studies provide information concerning the distributions of conformational states in the folded, unfolded, and dynamically interconverting states.
Figures
Similar articles
-
Thermal unfolding in a GCN4-like leucine zipper: 13C alpha NMR chemical shifts and local unfolding curves.Biophys J. 1997 Aug;73(2):1031-41. doi: 10.1016/S0006-3495(97)78136-0. Biophys J. 1997. PMID: 9251820 Free PMC article.
-
Energetics of coiled coil folding: the nature of the transition states.Biochemistry. 2001 Mar 27;40(12):3544-52. doi: 10.1021/bi002161l. Biochemistry. 2001. PMID: 11297420
-
Mapping the energy surface for the folding reaction of the coiled-coil peptide GCN4-p1.Biochemistry. 2001 Jan 23;40(3):719-31. doi: 10.1021/bi001438e. Biochemistry. 2001. PMID: 11170389
-
Rational design of a three-heptad coiled-coil protein and comparison by molecular dynamics simulation with the GCN4 coiled coil: presence of interior three-center hydrogen bonds.Protein Sci. 1994 Feb;3(2):345-55. doi: 10.1002/pro.5560030217. Protein Sci. 1994. PMID: 8003969 Free PMC article.
-
De novo design, synthesis and characterization of membrane-active peptides.Biochem Soc Trans. 2001 Aug;29(Pt 4):559-64. doi: 10.1042/bst0290559. Biochem Soc Trans. 2001. PMID: 11498028 Review.
Cited by
-
Development of a technique for the investigation of folding dynamics of single proteins for extended time periods.Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10453-8. doi: 10.1073/pnas.0700267104. Epub 2007 Jun 11. Proc Natl Acad Sci U S A. 2007. PMID: 17563378 Free PMC article.
-
Shot-noise limited single-molecule FRET histograms: comparison between theory and experiments.J Phys Chem B. 2006 Nov 9;110(44):22103-24. doi: 10.1021/jp063483n. J Phys Chem B. 2006. PMID: 17078646 Free PMC article.
-
Watching proteins fold one molecule at a time.Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3197-202. doi: 10.1073/pnas.2628068100. Epub 2003 Feb 28. Proc Natl Acad Sci U S A. 2003. PMID: 12612345 Free PMC article.
-
Polyproline and the "spectroscopic ruler" revisited with single-molecule fluorescence.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2754-9. doi: 10.1073/pnas.0408164102. Epub 2005 Feb 7. Proc Natl Acad Sci U S A. 2005. PMID: 15699337 Free PMC article.
-
DNA origami as biocompatible surface to match single-molecule and ensemble experiments.Nucleic Acids Res. 2012 Aug;40(14):e110. doi: 10.1093/nar/gks326. Epub 2012 Apr 20. Nucleic Acids Res. 2012. PMID: 22523083 Free PMC article.
References
-
- Xie X S, Trautman J K. Annu Rev Phys Chem. 1998;49:441–480. - PubMed
-
- Funatsu T, Harada Y, Tokunaga M, Saito K, Yanagida T. Nature (London) 1995;374:555–559. - PubMed
-
- Geva E, Skinner J L. Chem Phys Lett. 1998;288:225–229.
-
- Ishijima A, Kojima H, Funatsu T, Tokunaga M, Higuchi H, Tanaka H, Yanagida T. Cell. 1998;92:161–171. - PubMed
-
- Lu H P, Xun L, Xie X S. Science. 1998;282:1877–1882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

