Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells
- PMID: 11087863
- PMCID: PMC27189
- DOI: 10.1073/pnas.97.24.13126
Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells
Abstract
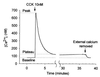
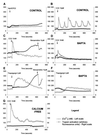
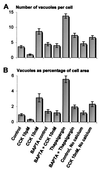
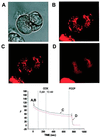
The pancreatic acinar cell produces powerful digestive enzymes packaged in zymogen granules in the apical pole. Ca(2+) signals elicited by acetylcholine or cholecystokinin (CCK) initiate enzyme secretion by exocytosis through the apical membrane. Intracellular enzyme activation is normally kept to a minimum, but in the often-fatal human disease acute pancreatitis, autodigestion occurs. How the enzymes become inappropriately activated is unknown. We monitored the cytosolic Ca(2+) concentration ([Ca(2+)](i)), intracellular trypsin activation, and its localization in isolated living cells with specific fluorescent probes and studied intracellular vacuole formation by electron microscopy as well as quantitative image analysis (light microscopy). A physiological CCK level (10 pM) eliciting regular Ca(2+) spiking did not evoke intracellular trypsin activation or vacuole formation. However, stimulation with 10 nM CCK, evoking a sustained rise in [Ca(2+)](i), induced pronounced trypsin activation and extensive vacuole formation, both localized in the apical pole. Both processes were abolished by preventing abnormal [Ca(2+)](i) elevation, either by preincubation with the specific Ca(2+) chelator 1, 2-bis(O-aminophenoxy)ethane-N,N-N',N'-tetraacetic acid (BAPTA) or by removal of external Ca(2+). CCK hyperstimulation evokes intracellular trypsin activation and vacuole formation in the apical granular pole. Both of these processes are mediated by an abnormal sustained rise in [Ca(2+)](i).
Figures



Comment in
-
Calcium signaling and acute pancreatitis: specific response to a promiscuous messenger.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):12933-4. doi: 10.1073/pnas.97.24.12933. Proc Natl Acad Sci U S A. 2000. PMID: 11087843 Free PMC article. Review. No abstract available.
References
-
- Palade G E. Science. 1975;189:347–358. - PubMed
-
- Case R M, Argent B E. In: Handbook of Physiology. Schultz S G, editor. Washington, DC: Am. Physiol. Soc.; 1989. pp. 383–417.
-
- Gorelick F S, Jamieson J D. In: Physiology of the Gastrointestinal Tract. 3rd Ed. Johnson L R, Alpers D H, Jacobson E D, Christensen J, Walsh J H, editors. New York: Raven; 1994. pp. 1353–1376.
-
- Neoptolemos J P. In: Acute Pancreatitis, in Bailliere's Clinical Gastroenterology. Neoptolemos J P, editor. Vol. 13. London: Bailliere Tindall; 1999.
-
- Kasai H. Trends Neurosci. 1999;22:88–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

