Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer
- PMID: 11087869
- PMCID: PMC27215
- DOI: 10.1073/pnas.97.24.13275
Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer
Abstract
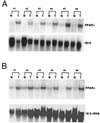
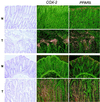
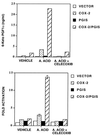
There is evidence from both genetic and pharmacologic studies to suggest that the cyclooxygenase-2 (COX-2) enzyme plays a causal role in the development of colorectal cancer. However, little is known about the identity or role of the eicosanoid receptor pathways activated by COX-derived prostaglandins (PG). We previously have reported that COX-2-derived prostacyclin promotes embryo implantation in the mouse uterus via activation of the nuclear hormone receptor peroxisome proliferator-activated receptor (PPAR) delta. In light of the recent finding that PPARdelta is a target of beta-catenin transactivation, it is important to determine whether this signaling pathway is operative during the development of colorectal cancer. Analysis of PPARdelta mRNA in matched normal and tumor samples revealed that expression of PPARdelta, similar to COX-2, is up-regulated in colorectal carcinomas. In situ hybridization studies demonstrate that PPARdelta is expressed in normal colon and localized to the epithelial cells at the very tips of the mucosal glands. In contrast, expression of PPARdelta mRNA in colorectal tumors was more widespread with increased levels in transformed epithelial cells. Analysis of PPARdelta and COX-2 mRNA in serial sections suggested they were colocalized to the same region within a tumor. Finally, transient transfection assays established that endogenously synthesized prostacyclin (PGI(2)) could serve as a ligand for PPARdelta. In addition, the stable PGI(2) analog, carbaprostacyclin, and a synthetic PPARdelta agonist induced transactivation of endogenous PPARdelta in human colon carcinoma cells. We conclude from these observations that PPARdelta, similar to COX-2, is aberrantly expressed in colorectal tumors and that endogenous PPARdelta is transcriptionally responsive to PGI(2). However, the functional consequence of PPARdelta activation in colon carcinogenesis still needs to be determined.
Figures


References
-
- Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Gastroenterology. 1994;107:1183–1188. - PubMed
-
- DuBois R N, Abramson S B, Crofford L, Gupta R A, Simon L S, Van De Putte L B, Lipsky P E. FASEB J. 1998;12:1063–1073. - PubMed
-
- Williams C S, Mann M, DuBois R N. Oncogene. 1999;18:7908–7916. - PubMed
-
- Gupta R A, DuBois R N. Gastroenterology. 1998;114:1095–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

