Acquired, nonrandom chromosomal abnormalities associated with the development of acute promyelocytic leukemia in transgenic mice
- PMID: 11087871
- PMCID: PMC27220
- DOI: 10.1073/pnas.97.24.13306
Acquired, nonrandom chromosomal abnormalities associated with the development of acute promyelocytic leukemia in transgenic mice
Abstract
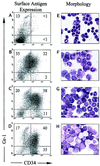
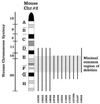
We previously generated a transgenic mouse model for acute promyelocytic leukemia (APL) by expressing the promyelocytic leukemia (PML)-retinoic acid receptor (RARalpha) cDNA in early myeloid cells. This fusion protein causes a myeloproliferative disease in 100% of animals, but only 15-20% of the animals develop acute leukemia after a long latency period (6-13 months). PML-RARalpha is therefore necessary, but not sufficient, for APL development. The coexpression of a reciprocal form of the fusion, RARalpha-PML, increased the likelihood of APL development (55-60%), but did not shorten latency. Together, these results suggested that additional genetic events are required for the development of APL. We therefore evaluated the splenic tumor cells from 18 transgenic mice with APL for evidence of secondary genetic events, by using spectral karyotyping analysis. Interstitial or terminal deletions of the distal region of one copy of chromosome 2 [del(2)] were found in 1/5 tumors expressing PML-RARalpha, but in 11/13 tumors expressing both PML-RARalpha and RARalpha-PML (P < 0.05). Leukemic cells that contained a deletion on chromosome 2 often contained additional chromosomal gains (especially of 15), chromosomal losses (especially of 11 or X/Y), or were tetraploid (P </= 0.001). These changes did not commonly occur in nontransgenic littermates, nor in aged transgenic mice that did not develop APL. These results suggest that expression of RARalpha-PML increases the likelihood of chromosome 2 deletions in APL cells. Deletion 2 appears to predispose APL cells to further chromosomal instability, which may lead to the acquisition of additional changes that provide an advantage to the transformed cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

