Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate-specific antigen levels
- PMID: 11089718
- PMCID: PMC1495603
- DOI: 10.1046/j.1525-1497.2000.90907.x
Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate-specific antigen levels
Abstract
Background: Prostate-specific antigen (PSA) levels between 4.0 to 10.0 ng/ml have poor specificity in prostate cancer screening, leading to unnecessary biopsies.
Objective: To determine whether the free-to-total PSA ratio (F/T PSA) improved the diagnostic accuracy of these nonspecific PSA levels.
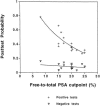
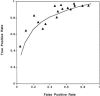
Measurements and main results: MEDLINE searchedwas from 1986 to 1997. Additional studies were identified from article bibliographies and by searching urology journals. Two investigators independently identified English-language studies providing F/T PSA ratio test-operating characteristics data on > or = 10 cancer patients with PSA values between 2.0 and 10.0 ng/ml. Twenty-one of 90 retrieved studies met selection criteria. Two investigators independently extracted data on methodology and diagnostic performance. Investigator-selected cut points for the optimal F/T PSA ratio had a median likelihood ratio of 1.76 (interquartile range, 1.40 to 2.11) for a positive test and 0.27 (0.20 to 0.40) for a negative test. Assuming a 25% pretest probability of cancer, the posttest probabilities were 37% following a positive test and 8% following a negative test. The summary receiver operating characteristic curve showed that maintaining test sensitivity above 90% was associated with false positive rates of 60% to 90%. Methodologic problems limited the validity and generalizability of the literature.
Conclusions: A negative test reduced the posttest probability of cancer to approximately 10%. However, patients may find that this probability is not low enough to avoid undergoing prostate biopsy. The optimal F/T PSA ratio cut point and precise estimates for test specificity still need to be determined.
Figures
Comment in
-
Early detection and aggressive treatment of prostate cancer: groping in the dark.J Gen Intern Med. 2000 Oct;15(10):749-51. doi: 10.1046/j.1525-1497.2000.00816.x. J Gen Intern Med. 2000. PMID: 11089719 Free PMC article. No abstract available.
References
-
- Mettlin C, Jones G, Averette H, Gusberg SB, Murphy GP, et al. Defining and updating the American Cancer Society guidelines for the cancer related check-up: prostate and endometrial cancers. CA Cancer J Clin. 1993;43:42–6. - PubMed
-
- American Urological Association. Early detection of prostate cancer and use of transrectal ultrasound. American Urological Association 1992 Policy Statement Book. Baltimore, Md: Williams & Wilkins; 1992.
-
- PDQ. (Physician Data Query) [database online]. Bethesda, Md: National Cancer Institute: 1984 – [updated 9/99]. Screening for prostate cancer. Available from: National Cancer Institute; National Library of Medicine, Bethesda, Md: CDP Technologies, Inc., New York, NY; Lexis-Nexis, Miamisburg, Ohio.
-
- American College of Physicians. Screening for prostate cancer. Ann Intern Med. 1997;126:480–4. - PubMed
-
- U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
