Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells
- PMID: 11090068
- PMCID: PMC2734459
Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells
Abstract
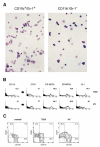
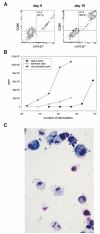
Apoptotic death of CD8(+) T cells can be induced by a population of inhibitory myeloid cells that are double positive for the CD11b and Gr-1 markers. These cells are responsible for the immunosuppression observed in pathologies as dissimilar as tumor growth and overwhelming infections, or after immunization with viruses. The appearance of a CD11b(+)/Gr-1(+) population of inhibitory macrophages (iMacs) could be attributed to high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) in vivo. Deletion of iMacs in vitro or in vivo reversed the depression of CD8(+) T-cell function. We isolated iMacs from the spleens of immunocompromised mice and found that these cells were positive for CD31, ER-MP20 (Ly-6C), and ER-MP58, markers characteristic of granulocyte/monocyte precursors. Importantly, although iMacs retained their inhibitory properties when cultured in vitro in standard medium, suppressive functions could be modulated by cytokine exposure. Whereas culture with the cytokine interleukin 4 (IL-4) increased iMac inhibitory activity, these cells could be differentiated into a nonadherent population of fully mature and highly activated dendritic cells when cultured in the presence of IL-4 and GM-CSF. A common CD31(+)/CD11b(+)/Gr-1(+) progenitor can thus give rise to cells capable of either activating or inhibiting the function of CD8(+) T lymphocytes, depending on the cytokine milieu that prevails during antigen-presenting cell maturation. (Blood. 2000;96:3838-3846)
Figures






References
-
- Sprent J, Schaefer M. Antigen-presenting cells for unprimed T cells. Immunol Today. 1989;10:17–23. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. - PubMed
-
- Lenardo M, Chan KM, Hornung F, et al. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
