Ku acts in a unique way at the mammalian telomere to prevent end joining
- PMID: 11090128
- PMCID: PMC317061
- DOI: 10.1101/gad.844000
Ku acts in a unique way at the mammalian telomere to prevent end joining
Abstract
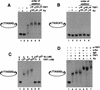
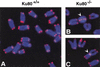
Telomeres are specialized DNA/protein structures that act as protective caps to prevent end fusion events and to distinguish the chromosome ends from double-strand breaks. We report that TRF1 and Ku form a complex at the telomere. The Ku and TRF1 complex is a specific high-affinity interaction, as demonstrated by several in vitro methods, and exists in human cells as determined by coimmunoprecipitation experiments. Ku does not bind telomeric DNA directly but localizes to telomeric repeats via its interaction with TRF1. Primary mouse embryonic fibroblasts that are deficient for Ku80 accumulated a large percentage of telomere fusions, establishing that Ku plays a critical role in telomere capping in mammalian cells. We propose that Ku localizes to internal regions of the telomere via a high-affinity interaction with TRF1. Therefore, Ku acts in a unique way at the telomere to prevent end joining.
Figures
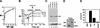
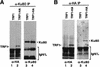

References
-
- Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. - PubMed
-
- Blackburn E. The telomere and telomerase: How do they interact? Mt Sinai J Med. 1999;66:292–300. - PubMed
-
- Blackburn EH. The telomere and telomerase: Nucleic acid-protein complexes acting in a telomere homeostasis system. A review. Biochemistry. 1997;62:1196–1201. - PubMed
-
- Bodnar AG, Ouellete M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
