Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs
- PMID: 11090146
- PMCID: PMC112429
- DOI: 10.1128/jvi.74.24.11495-11503.2000
Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs
Abstract
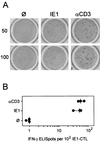
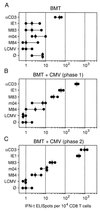
Interstitial cytomegalovirus (CMV) pneumonia is a clinically relevant complication in recipients of bone marrow transplantation (BMT). Recent data for a model of experimental syngeneic BMT and concomitant infection of BALB/c mice with murine CMV (mCMV) have documented the persistence of tissue-resident CD8 T cells after clearance of productive infection of the lungs (J. Podlech, R. Holtappels, M.-F. Pahl-Seibert, H.-P. Steffens, and M. J. Reddehase, J. Virol. 74:7496-7507, 2000). It was proposed that these cells represent antiviral "standby" memory cells whose functional role might be to help prevent reactivation of latent virus. The pool of pulmonary CD8 T cells was composed of two subsets defined by the T-cell activation marker L-selectin (CD62L): a CD62L(hi) subset of quiescent memory cells, and a CD62L(lo) subset of recently resensitized memory-effector cells. In this study, we have continued this line of investigation by quantitating CD8 T cells specific for the three currently published antigenic peptides of mCMV: peptide YPHFMPTNL processed from the immediate-early protein IE1 (pp89), and peptides YGPSLYRRF and AYAGLFTPL, derived from the early proteins m04 (gp34) and M84 (p65), respectively. IE1-specific CD8 T cells dominated in acute-phase pulmonary infiltrates and were selectively enriched in latently infected lungs. Notably, most IE1-specific CD8 T cells were found to belong to the CD62L(lo) subset representing memory-effector cells. This finding is in accordance with the interpretation that IE1-specific CD8 T cells are frequently resensitized during latent infection of the lungs and may thus be involved in the maintenance of mCMV latency.
Figures


References
-
- Alterio de Goss M, Holtappels R, Steffens H-P, Podlech J, Angele P, Dreher L, Thomas D, Reddehase M J. Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells. J Virol. 1998;72:7733–7744. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

